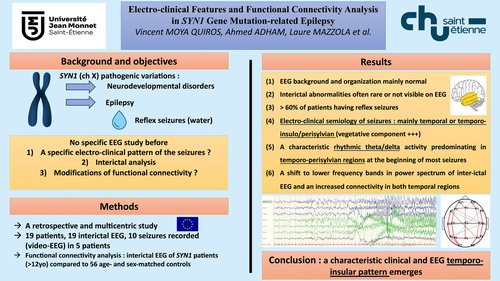
Electro-Clinical Features and Functional Connectivity Analysis in SYN1-Related Epilepsy
Abstract
Objective
There is currently scarce data on the electroclinical characteristics of epilepsy associated with synapsin 1 (SYN1) pathogenic variations. We examined clinical and electro-encephalographic (EEG) features in patients with epilepsy and SYN1 variants, with the aim of identifying a distinctive electroclinical pattern.
Methods
In this retrospective multicenter study, we collected and reviewed demographic, genetic, and epilepsy data of 19 male patients with SYN1 variants. Specifically, we analyzed interictal EEG data for all patients, and electro-clinical data from 10 epileptic seizures in 5 patients, using prolonged video-EEG monitoring recordings. Inter-ictal EEG functional connectivity parameters and frequency spectrum of the 10 patients over 12 years of age, were computed and compared with those of 56 age- and sex-matched controls.
Results
The main electroclinical features of epilepsy in patients with SYN1 were (1) EEG background and organization mainly normal; (2) interictal abnormalities are often rare or not visible on EEG; (3) more than 60% of patients had reflex seizures (cutaneous contact with water and defecation being the main triggers) isolated or associated with spontaneous seizures; (4) electro-clinical semiology of seizures was mainly temporal or temporo-insulo/perisylvian with a notable autonomic component; and (5) ictal EEG showed a characteristic rhythmic theta/delta activity predominating in temporo-perisylvian regions at the beginning of most seizures. Comparing patients with SYN1 to healthy subjects, we observed a shift to lower frequency bands in power spectrum of interictal EEG and an increased connectivity in both temporal regions.
Interpretation
A distinct epilepsy syndrome emerges in patients with SYN1, with a rather characteristic clinical and EEG pattern suggesting predominant temporo-insular involvement. ANN NEUROL 2025;97:34–50
Graphical Abstract
Synapsins are a group of presynaptic phosphoproteins predominantly expressed in the central and peripheral nervous systems.1 They have a crucial role in synaptogenesis and synaptic plasticity.1, 2 Synapsin 1 (SYN1) gene codes for 1 of the 3 synapsins. Pathogenic variations in SYN1 are associated with X-linked neurodevelopmental disorders mainly affecting male subjects, with clinical heterogeneity and intrafamilial variability. Currently, 85 (73 male and 12 female) cases have been reported in 50 families.2-22 Epilepsy, speech delay, learning disabilities, intellectual disability (ID), autism spectrum disorder (ASD), and attention-deficit/hyperactivity disorder (ADHD) are the most frequent clinical features associated with SYN1 pathogenic variations.15, 21, 23, 24
Epilepsy is one of the main features reported in recent studies that concern up to 82% of patients with SYN1 variants.21 The presence of reflex seizures is one of the most distinctive clinical manifestations. In more than half of the described cases, seizures are triggered by cutaneous contact with water, such as bathing or showering. Other triggers are also mentioned, such as rubbing with a towel, haircutting, fingernail clipping, mental representation of bathing, toothbrushing, defecating, and laughing.3, 5, 15, 19-21, 25, 26 Spontaneous seizures are frequently associated and psychogenic non-epileptic seizures (PNES) are reported.5, 20
Despite this knowledge about the general characteristics of epilepsy related to SYN1, comprehensive data focusing on electroclinical features are scarce, imprecise, and sometimes contradictory. The type of epilepsy is not sufficiently well characterized, with variable seizure types reported, both focal and generalized, clinical descriptions being mostly vague and succinct, based mainly on family and caregiver reports. Very few seizures have been documented by video-EEG. Ictal video-EEG data are reported in only 6 patients,5, 10, 15, 25 and ictal EEG from an ambulatory home EEG in 1 patient.26 To our knowledge, no study has specifically examined the EEG features of epilepsies associated with SYN1 variants. In this study, we report EEG data from 19 patients with SYN1-related epilepsy. Video-EEG data from 10 seizures in 5 patients were analyzed and compared with literature data, with the aim of identifying a characteristic electroclinical pattern. We also described the principal interictal EEG abnormalities, their type, and distribution.
As brain connectivity organization can be affected in patients with epilepsy,27-32 we also studied the interictal functional connectivity of these patients and compared it with data from 56 age- and sex-matched controls (for patients over 12 years of age). The epileptic networks in focal epilepsies are mostly associated to a focal increase of functional connectivity markers, and a global decrease is linked to neurocognitive disorders.27, 29, 32 We aimed to determine if connectivity networks would be affected by the epileptic process and/or altered due to the neurocognitive disorders found in patients with SYN1-related epilepsy. Thus, we compared brain connectivity maps and network connectivity parameters between patients with SYN1 variants and a matched group of healthy control subjects. We also analyzed the interictal EEG power-spectrum.
We aimed to further delineate the electroclinical patterns of individuals with SYN1 pathogenic variations, with the goal of characterizing interictal and ictal EEG patterns. Our findings will help clinicians to diagnose SYN1-related epilepsy earlier, to better understand the underlying pathophysiological mechanisms which would improve future management.
Methods
Participant Recruitment
Patients With SYN1 Pathogenic Variations
All patients met the following inclusion criteria: (1) having a likely pathogenic or pathogenic variation of SYN1 identified by gene panels or exome sequencing; (2) presenting with epilepsy (related to the SYN1 variant); (3) having benefited from EEG recordings with interictal +/− video-EEG ictal data available for review; and (4) availability of detailed clinical data, including neurodevelopmental assessment, neuropsychological and behavioral tests, brain magnetic resonance imaging, epilepsy phenotype, and response to treatment.
Recruitment was made through announcements in epilepsy networks, such as the European Reference Network for rare and complex epilepsies (ERN EpiCARE) and French League Against Epilepsy (LFCE). We also contacted the authors of the largest patient series with SYN1 variants, published recently, and focusing on genotype/phenotype correlations, to ask if EEG data were available.21 All procedures were performed respecting the ethical standards of the institutional and/or national research committees, and in conformity with the 1964 Helsinki Declaration or comparable ethical standards. Data collection was approved by the University Hospital of Saint-Etienne Medical Ethics Review Committee (IRBN662023/CHUSTE). Patients and/or legal guardians were informed about inclusion in the study.
A total of 19 patients were included. Five patients were documented for the first time, and 14 had already been reported in another study.15, 21 They came from 13 different centers located in 5 European countries. Ictal video-EEG was available for 5 patients and data from 10 seizures were analyzed.
Controls for Interictal Functional Connectivity Analysis
The 56 control subjects had a standard EEG recording performed with 19 electrodes (Micromed, Treviso, Italy) at the University Hospital of Saint-Etienne. They were matched to the patients for age and gender. They were selected from our EEG database. The healthy controls had no history of epilepsy, neurologic disorders, EEG, or neuro-imaging abnormalities. Taking into account that the connectivity networks evolve during the first years of life, thus making interpretation difficult, healthy subjects were all aged over 12 years old.33 Patients with SYN1 pathogenic variations younger than 12 years old were excluded from this analysis. EEG of control subjects had to include at least 5 minutes of artifact-free open-eyed resting recording. The analysis could so be performed in 10 patients.
Demographic, Genetic, and Epilepsy Data
After anonymization of patients’ data, each referring physician provided detailed neurological, developmental, and behavioral history. We collected information about the age at the time of study, the sex, and the characteristics of the SYN1 variants. Regarding comorbidities, we assessed ID, ASD, and ADHD, other behavioral issues, and learning difficulties. Related to the epilepsy, we investigated the age at seizure onset, the seizure type(s) at onset and during disease course (as described by family and caregivers), the history of febrile seizures and status epilepticus, the seizure frequency, the presence of reflex seizures and the triggers involved, the occurrence or not of spontaneous seizures, the previous and current anti-seizure medications, and overall response to treatment.
Receiver operating characteristics (ROC) and area under the curve (AUC) analyses were used to assess if the age at epilepsy onset could be predictive of ID or of response to antiseizure medications. The possible association between having an ID and a drug-resistant epilepsy was assessed using a Cochran–Armitage test.
EEG Data
Inter-Ictal and Ictal EEG Data Recordings
Patients’ EEG data were recorded in each participating center, then anonymized and sent in a Micromed, Natus, or edf format for analysis. EEGs were obtained using 19 electrodes placed according to the 10/20 international system (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, Cz, and Pz), or using 11 electrodes (Fp1, Fp2, C3, C4, F7, F8, T5, T6, O1, O2, and Cz) and a reference. Signal was acquired at 256 Hz sampling, amplified (1,000 times) and filtered to the 0.01 to 97 hertz (Hz) band. Interictal EEG features and ictal patterns analysis were performed by 2 neurophysiologists/epileptologists who completed an EEG reading chart for systematic data collection.
Electro-cardiographic modifications during seizures were also evaluated. Tachycardia was defined as an RR interval decrease over the baseline mean – 1 standard deviation (SD), and bradycardia as a RR interval increase over the baseline mean + 1 SD.34
Inter-Ictal Functional Connectivity Data Analysis
Inter-ictal EEG analysis was done in MATLAB 2019b, with the EEGLAB Toolbox. Continuous EEG recordings were bandpass-filtered between 0.01 and 97 Hz using a finite impulse response filter. A Notch filter was also applied at 50 Hz to remove line noise artifact. The data were referenced to infinite source with the REST algorithm.35 A manual slicing of the EEG was then realized by a neurophysiologist to eliminate time periods with muscle artifacts. Eye artifacts were also removed manually.
At first, we computed simple Fourier's transformation to extract the spectral power for each electrode, for each patient (n = 10) and for each frequency band. We then averaged the data respectively for each group.
For connectivity computation, the broadband time-domain source signals were band-pass filtered at 10 Hz (± 2 Hz). A space Laplacian filter was applied to the data to minimize the effects of volume conduction. A Hilbert transform was then applied, before assessing connectivity with the Phase Locking Value (PLV). The PLV is a normalized value, that gives for each pair of electrodes a value ranging from 0 (no phase locking) to 1 (complete phase locking). For visual comparison and plotting, we then eliminated links that were weaker than 1 SD from the mean PLV value, which kept only the strongest links.
Some important markers of connectivity inside the network are the clustering coefficient and the path length value.36, 37 The path length refers to the minimal number of edges that must be traversed to travel from one node in the network to another and the clustering coefficient is defined as the connection probability of nearest neighbor nodes. A short average path length and a high average clustering coefficient are correlated to an efficient brain network. We computed the average path length and clustering coefficient from the connectivity PLV matrixes, thanks to the toolbox BCT in MatLAB.38, 39
Results
Clinical and Genetic Data
Our 19 patients came from 16 different families. Patients VIII-1 and VIII-2, as well as II-1, II-2, and II-3 were, respectively, brothers. Demographic, clinical, and genetic data are summarized in Tables 1 to 3.
| Patient | I-1a | II-1a | II-2a | II-3a | III-1 | IV-1a | V-1a |
|---|---|---|---|---|---|---|---|
| Age at the time of the study, gender | 11 yr, M | 16 yr, M | 21 yr, M | 19 yr, M | 29 yr, M | 11 yr, M | 15 yr, M |
| City/country of collected data | Saint-Etienne/France | Saint-Etienne/France | Saint-Etienne/France | Saint-Etienne/France | Lyon/France | Lyon and Dijon/France | Lille/France |
| Mutation type | Frameshift | Nonsense | Nonsense | Nonsense | Frameshift | Frameshift | Missense |
| SYN1 variant; (NM_006950.3); inheritance | c.1321dup; p.(Ala441Glyfs*243); Mat | c.1447C > T; p.(Gln483*); Mat | c.1447C > T; p.(Gln483*); Mat | c.1447C > T; p.(Gln483*); Mat | c.122del; p.(Gly41Glufs*103); Mat | c.1794_1906del; p.(Thr601Glufs*45); Mat | c.774G > T; p.(Met258Ile); Mat |
| Development - learning disabilities | Moderate language delay | Language delay, executive disorder, multi days | Language delay, multi days | Executive disorder, multi days | Normal | Severe ID | Mild ID |
| Behavioral disturbances | Intolerance to frustration | No | ADHD | No | No | ASD | Impulsive behavior |
| Neurological examination abnormalities | No | Tics | No | No | No | Stereotypies, hypotonia, ataxia | No |
| Neuroimaging abnormalities (MRI) | No | No | No | No | No | No | No |
| Age at seizure onset | 10 mo | 11 yr | 7 yr | 13 yr | 6 yr | 6 mo | 11 yr |
| Suspected seizure type based on medical reports | Focal with rare secondary GTCS | Focal with rare secondary GTCS | Focal with frequent secondary GTCS | Focal with possible secondary GTCS | Focal with rare secondary GTCS | Focal with possible secondary GTCS | Focal sensory |
| Presence of reflex seizures/triggers | Yes/bathing | Yes/showering with warm water | Yes/showering with warm water | Yes/showering with warm water | Yes/defecating | No | Yes |
| SS, FS, and SE | SS and FS | SS | SS | SS | SS | SS | No |
| Frequency of seizures | Once a week | Once a week | Once a week | Once a week | Stopped with treatment | Daily | Only once |
| Current/Previous anti-seizure medications | VPA, LTG, CBD/CLB, CBZ, LEV | LEV/CLB, LTG | OXC/BRV, CLB, LAC, VPA | LTG/CLB | OXC, LEV, LAC/CBZ, PER, ZNS | ZNS, VPA, CLN/TPM, LEV | No |
| Drug resistance | Yes | Yes | Yes | Yes | No | Yes | No |
| Interictal EEG | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ictal EEG/Number of seizures | Yes/3 | Yes/1 | No | No | Yes/1 | Yes/2 | No |
- ADHD = attention-deficit/hyperactivity disorder; ASD = autism spectrum disorder; BRV = brivaracetam; CBD = cannabidiol; CBZ = carbamazepine; CLB = clobazam; CLN = clonazepam; DN = de novo; ECB = eslicarbazepine; F = female; FS = febrile seizures; GTCS = generalized tonic–clonic seizures; ID = intellectual disability; LAC = lacosamide; LEV = levetiracetam; LTG = lamotrigine; M = male; Mat = maternal; mo = months; MRI = magnetic resonance imaging; MVD = moderate ventricular dilatation; NA = not available; OXC = oxcarbazepine; TPM = topiramate; PER = perampanel; SE = status epilepticus; SS = spontaneous seizures; STM = sulthiame; VGB = vigabatrin; VPA = valproic acid; y = years; ZNS = zonisamide.
- a Refers to patients yet published in the following article: Parenti I, Leitão E, Kuechler A, et al. The different clinical facets of SYN1-related neurodevelopmental disorders. Front Cell Dev Biol 2022; 10: 1019715.
- b Refers to patients yet published in the following article: Accogli A, Wiegand G, Scala M, et al. Clinical and Genetic Features in Patients With Reflex Bathing Epilepsy. Neurology 2021; 97 (6): e577–e586.
| Patient | VI-1a | VII-1a | VIII-1a | VIII-2a | IX-1 | X-1 |
|---|---|---|---|---|---|---|
| Age at the time of the study, gender | 28 yr, M | 9 yr, M | 17 yr, M | 32 yr, M | 10 yr, M | 20 yr, M |
| City/country of collected data | Lille/France | Strasbourg/France | Montpellier/France | Libourne/France | Genova/Italy | Bielefeld/Germany |
| Mutation type | Missense | Frameshift | Frameshift | Frameshift | Splice | Splice |
| SYN1 variant; (NM_006950.3); inheritance | c.614 T > A; p.(Leu205Gln); Mat | c.39del; p.(Phe13Leufs*10); Mat | c.975del; p.(Tyr326Thrfs*2); Mat | c.975del; p.(Tyr326Thrfs*2); Mat | c.527 + 1G > T; Mat | c.838-2A > G; Mat |
| Development - learning disabilities | Normal | Moderate ID | Language delay, dyslexia | Normal | Mild ID, language delay | Executive disorder |
| Behavioral disturbances | Impulsive behavior | Anxiety, hyperkinetic | ADHD | Aggressive behavior | No | ADHD |
| Neurological examination abnormalities | No | No | No | No | Stereotypies | No |
| Neuroimaging abnormalities (MRI) | No | No | Enlarged Virchow-Robin | MVD, small hypophysis | No | No |
| Age at seizure onset | 11 yr | 1 y 6 mo | 3 yr | 7 yr | 5 yr | 7 yr |
| Suspected seizure type based on medical reports | GTCS | Focal with possible secondary GTCS | Myoclonic | GTCS | Behavior arrest of unknown onset | GTCS |
| Presence of reflex seizures/triggers | Yes/defecating | Yes/stroboscope | No | Yes/emotions and lightning | No | No |
| SS, FS, and SE | SS and FS | SS and FS | SS and FS | SS | SS | SS |
| Frequency of seizures | Stopped with treatment | NA | Only once | 1–2 seizures/yr | Monthly | Stopped with treatment |
| Current/previous antiseizure medications | LTG, OXC | LEV | VPA | LTG/VGB, VPA | LEV, VPA | LAC |
| Drug resistance | No | No | No | Yes | Yes | No |
| Interictal EEG | Yes | Yes | Yes | Yes | Yes | Yes |
| Ictal EEG/number of seizures | No | No | No | No | No | No |
- ADHD = attention-deficit/hyperactivity disorder; EEG = encephalographic; FS = febrile seizures; GTCS = generalized tonic–clonic seizures; ID = intellectual disability; LAC = lacosamide; LEV = levetiracetam; LTG = lamotrigine; mo = months; MRI = magnetic resonance imaging; MVD = moderate ventricular dilatation; NA = not available; OXC = oxcarbazepine; SE = status epilepticus; SS = spontaneous seizures; VGB = vigabatrin; VPA = valproic acid; y = years.
| Patient | XI-1b | XII-1b | XIII-1 | XIV-1a | XV-1 | XVI-1a, b |
|---|---|---|---|---|---|---|
| Age at the time of the study, gender | 5 yr 6 mo, M | 2 yr 6 mo, M | 1 yr 9 mo, M | 14 yr, M | 13 yr, M | 4 yr, M |
| City/country of collected data | Hamburg, Germany | Hamburg, Germany | Heeze, The Netherlands | Philadelphia/Denmark | Toulouse, France | Nijmegen, The Netherlands |
| Mutation type | Frameshift | Frameshift | Frameshift | Frameshift | Inframe deletion | Frameshift |
| SYN1 variant, (NM_006950.3), inheritance | c.1472_1473insT; p.(Gln491Hisfs*193); Mat | c.1406dupA; p.(Pro470Alafs*214); Mat | c.1779dup; p.(Pro594ThrfsTer90); DN | c.39del; p.(Phe13Leufs*10); Mat | c.90_104del; p.Pro31_Pro35del; Mat | c.1647_1650dup; p.(Ser551fs); DN |
| Development - learning disabilities | Moderate ID | Mild ID, language delay | Language delay | Mild ID | Severe ID | Difficulty with pronunciation, language delay |
| Behavioral disturbances | ASD, ADHD | ADHD | No | Borderline behavior | ASD | ADHD, hypersensitivity |
| Neurological examination abnormalities | No | No | No | No | Hypertonia and hyperreflexia | No |
| Neuroimaging abnormalities (MRI) | No | No | No | Minor multicystic corpus pineal cyst | No | No |
| Age at seizure onset | 4 yr 6 mo | 2 yr | 1 yr 6 mo | 10 yr | 2 yr | 3 yr 6 mo |
| Suspected seizure type based on medical reports | Focal non motor autonomic with possible secondary GTCS | Focal with impaired awareness and possible secondary GTCS | Focal automatisms and impaired awareness with possible secondary GTCS | Focal autonomic onset with automatisms and impaired awareness | GTCS, atonic, myoclonic | Focal with possible secondary GTCS |
| Presence of reflex seizures/triggers | Yes/bathing and showering, haircutting, fingernail clipping, idea of bathing | Yes/bathing | No | Yes/toothbrushing, bathing and showering | No | No |
| SS, FS, and SE | SS and SE | SS | SS, FS, and SE | No | SS and FS | SS and SE |
| Frequency of seizures | 2–8/mo | Weekly | Clustering when he is ill | 1–2/mo | Stopped with treatment | Stopped with treatment |
| Current/previous antiseizure medications | VPA, LTG, STM | VPA | LEV, VPA/CBZ | ZNS, CBZ/ECB | VPA/TPM, CLB, LEV | LEV |
| Drug resistance | Yes | Yes | Yes | Yes | No | No |
| Interictal EEG | Yes | Yes | Yes | Yes | Yes | Yes |
| Ictal EEG/number of seizures | Yes/3 | No | No | No | No | No |
- ADHD = attention-deficit/hyperactivity disorder; ASD = autism spectrum disorder; CBZ = carbamazepine; CLB = clobazam; DN = de novo; ECB = eslicarbazepine; EEG = encephalographic; FS = febrile seizures; GTCS = generalized tonic–clonic seizures; ID = intellectual disability; LEV = levetiracetam; LTG = lamotrigine; M = male; Mat = maternal; mo = months; MRI = magnetic resonance imaging; TPM = topiramate; SE = status epilepticus; SS = spontaneous seizures; STM = sulthiame; VPA = valproic acid; yr = years.
SYN1 variant types included frameshift (n = 11; 58%), nonsense (n = 3; 16%), missense (n = 2; 11%), splice site (n = 2; 11%), or inframe deletion (n = 1; 5%). Eighty-four percent (n = 16/19) of patients had truncating variants of which 19% (n = 3/16) had a moderate or severe ID, and 16% (n = 3/19) had non-truncating variants among whom 33% (n = 1/3) had a moderate or severe ID (Fig 1). All individuals had maternal inheritance, except for 2 patients with a de novo variation. Medical information was available for the large majority of carrier mothers’, none being reported as suffering from epilepsy.
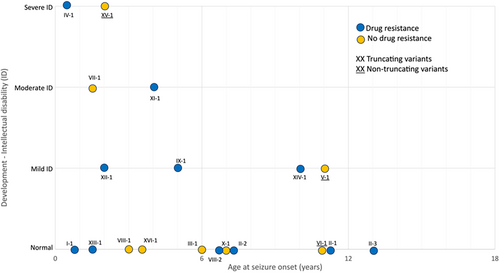
All patients were male subjects. The mean age at last follow up was 14.6 years (range from 21 months to 32 years old). A total of 8 of 19 patients (42%) had an ID ranging from mild (n = 4), moderate (n = 2), to severe (n = 2). Learning disabilities were reported in 16 patients (84%). All patients younger than 5 years old (n = 3) presented with a language delay. ASD was diagnosed in 16% (3/19) and ADHD in 32% (6/19). No major neuroimaging abnormality was noticed.
The onset of epileptic seizures ranged from 6 months to 13 years of life, with a median age at onset of 4.5 years. In 11% of cases (n = 2), seizure-onset occurred before the second year of life, and in 47% (n = 9) within the first 5 years of age. An early age of seizure onset (before 5 years old) was associated with an increased risk for moderate to severe ID (p < 0.036, AUC = 85%, IC = 70 à 100%). Sixty percent (n = 6/10) of the patients with epilepsy onset before the age of 5 years presented with an associated ID versus 22% (n = 2/9) in the rest of the cohort (see Fig 1). An age under 10 years at seizure onset was found in 81% (n = 13/16) of the patients with truncating variants and in 33% (n = 1/3) of those with non-truncating variants.
Reported Seizures Symptoms
The types of seizures, as reported by families and caregivers, were focal in 68% (n = 13) with possible secondary generalization in 58% (n = 11). Five patients were described as having generalized seizures (tonic–clonic [n = 4], myoclonic [n = 2], atonic [n = 1] and a behavioral arrest of unknown origin in 1 patient). Autonomic manifestations were described in 11% of the individuals (n = 2), somatosensory manifestations in 5% (n = 1), behavioral arrest in 5% (n = 1), and impaired awareness in 16% (n = 3). Some descriptions were succinct, particularly for seizures in infants and children who cannot verbally express the sensations experienced during their seizures.
Altogether, 63% (n = 12/19) of the reported seizures presented a reflex component. Triggers were showering or bathing (n = 7), defecating (n = 2), emotions (n = 1), lightning strikes (n = 1), haircutting (n = 1), fingernail clipping (n = 1), the idea of bathing (n = 1), and toothbrushing (n = 1). Bathing reflex seizures were provoked by warm water in 3 patients and regardless of the temperature of the water in 4 patients. The combination of provoked and unprovoked seizures was frequent during the course of epilepsy, present in 53% (n = 10) of the patients. Thirty-seven percent of the patients (n = 7) had only unprovoked seizures. Thirty-two percent (n = 6) of the patients had a history of febrile seizures.
Fifty-eight percent of the patients (n = 11) proved to be drug-resistant; all had a truncating variant. The age at epilepsy onset was not predictive of response to antiseizure medications (p = 0.68, AUC = 44%, NS) and drug-resistance was not statistically associated with ID (p = 0.10, NS, Cochran–Armitage test). Learning disabilities or behavioral disturbances were found in 86% (n = 6) of patients who were seizure-free and in all other patients.
Video-EEG Recorded Seizures
We analyzed the video-EEG data of 10 epileptic seizures recorded in 5 patients. For one patient (III-1), the seizure occurred while he was on the toilet (defecating), consequently the videotaping was not on, and the description was based on the nurse's report.
The clinical semiology of recorded seizures and EEG findings are summarized in Tables 4 to 7. The clinical sequence of events most frequently observed during seizures were associated orofacial automatisms (chewing), impaired awareness, and autonomic manifestations, such as pallor, cyanosis, or changes in cardiac rhythm. Oro-facial automatisms were seen at the onset or occurred very early in 4 of 10 of the video-EEG recorded seizures.
| Patient | I-1a | II-1a | III-1 |
|---|---|---|---|
| Awake interictal | |||
| Background activity | Slow (theta) | Normal (alpha) | Normal (alpha) |
| Spatial organization | Correct | Correct | Correct |
| Paroxystic GE | Rare L T S | Rare R F-T S | No |
| Slow waves | Rare/L T | No | No |
| Photosensitivity | No | No | No |
| Sleep interictal | |||
| Physiologic GE during sleep | Yes | Yes | Yes |
| Paroxystic GE | Rare L T S | R F-T S | No |
| Slow waves | Rare/L T | No | No |
| Sleep S activation | No | Yes | No |
| Recorded seizures | 1 | 2 | 3 | 1 | 1 |
|---|---|---|---|---|---|
| Clinical description | OFA, then ipsilateral (L) eye blinking and impaired awareness, then R upper limb dystonia, post ictal confusion | OFA, then left moderate upper limb dystonia and cough | OFA and bilateral eye blinking, then bilateral upper limbs dystonia predominant on the R at last confusion | OFA and bad feeling, then rapid L eye blinking and repetitive R upper limb automatisms, at last secondary generalization | Impaired awareness during defecation, then R upper limb automatisms, OFA, hypersalivation, pallor/sweating, and fall |
| Ictal EEG description | Fast low voltage activity then rhythmic theta/delta activity in L T regions evolving in rhythmic SW. Then diffusion to both subsylvian regions. L post ictal slowing | Short fast low voltage activity then rhythmic theta/delta activity in R T regions evolving in rhythmic SW diffusing in the R hemisphere. R post ictal slowing | Rhythmic theta and delta activity in L T regions, then propagating in R T regions, after bilateral T rhythmic SW discharge. Diffuse post ictal slowing activity | Rhythmic theta activity in R T region evolving in R hemispheric rhythmic SW and then secondary generalization followed by global depression. R F-T post ictal slowing | R T-B rhythmic theta activity evolving to theta delta activity in bilateral F-T regions, predominant on the R hemisphere. R F-T post ictal slowing |
| ECG | Tachycardia | Tachycardia | Tachycardia | Tachycardia | Tachycardia |
| Seizure onset | L temporo-mesial | R temporo-mesial | L temporo-mesial | R temporo-mesial | R temporo-insular |
- B = basal; C = central; EEG = encephalographic; F = frontal; GE = grapho-element; L = left; O = occipital; OFA = oro-facial automatisms; R = right; S = spike; SW = spike waves; T = temporal.
- a Refers to patients yet published in the following article: Parenti I, Leitão E, Kuechler A, et al. The different clinical facets of SYN1-related neurodevelopmental disorders. Front Cell Dev Biol 2022; 10: 1019715.
- b Refers to patients yet published in the following article: Accogli A, Wiegand G, Scala M, et al. Clinical and Genetic Features in Patients With Reflex Bathing Epilepsy. Neurology 2021; 97 (6): e577–e586.
| Patient | IV-1a | XI-1b |
|---|---|---|
| Awake interictal | ||
| Background activity | Normal (alpha) | Normal (alpha) |
| Spatial organization | Diffusing | Correct |
| Paroxystic GE | BiT asynchronous S, SW | Frequent R T SW |
| Slow waves | No | No |
| Photosensitivity | No | No |
| Sleep interictal | ||
| Physiologic GE during sleep | Yes | Yes |
| Paroxystic GE | BiT asynchronous S, SW | Frequent and ample R T S and SW |
| Slow waves | No | No |
| Sleep S activation | Yes | Yes |
| Recorded seizures | 1 | 2 | 1 | 2 | 3 |
|---|---|---|---|---|---|
| Clinical description | Slow L deviation of head/eyes, then upper limbs hypertonia and L clonic head deviation and L upper limb clonies | Left gaze deviation, then bilateral crispation of the corners of the mouth. At last R hemibody hypomobility | Asymptomatic for 1 min while bathing, then pallor, apnea, cyanosis and no response, at last L head/eyes deviation | Chills and cyanosis after bathing, then difficulty to answer questions and OFAs | Impaired awareness during bathing, cyanosis/OFA, head/limbs hypotonia, then shoulders hypertonia and cough |
| Ictal EEG description | BiF rhythmic theta activity (predominating in R F region) and then secondary generalization | Rhythmic theta-delta activity in L central region evolving rhythmic SW in L T region | R C-T sharp waves, then R C-T theta rhythmic activity second diffusing, at last diffuse depression of EEG activity | Diffuse rhythmic theta activity, predominantly in R F-T region, then diffuse rhythmic high amplitude delta activity | Enhancement of interictal R T SW, then theta rhythmic activity in R T region evolving to rhythmic SW activity in R hemisphere and then in both hemispheres |
| ECG | Tachycardia | Tachycardia | Bradycardia | Tachycardia | Tachycardia |
| Seizure onset | R frontal | L frontal | R temporo-insular | R fronto-temporal | R temporal |
- BiF = Bi-frontal; BiT = Bi-temporal; C = central; EEG = encephalographic; F = frontal; GE = grapho-element; L = left; O = occipital; OFA = oro-facial automatisms; R = right; S = spike; SW = spike waves; T = temporal.
| Patient | IX-1 | X-1 | XII-1b | XIII-1 | XIV-1a | XV-1 | XVI-1a, b |
|---|---|---|---|---|---|---|---|
| Awake interictal | |||||||
| Background activity | Normal (alpha) | Normal (alpha) | Normal (alpha) | Normal (theta-alpha) | Normal (alpha) | Normal (alpha) | Normal (alpha) |
| Spatial organization | Correct | Correct | Correct | Correct | Correct | Correct | Correct |
| Paroxystic GE | Rare R F-C-T S and SW | R F S | No | No | No | No | No |
| Slow waves | No | No | No | Yes/L hemisphere with C-T predominance | No | Yes/diffuse | No |
| Photosensitivity | No | No | No | No | No | No | No |
| Sleep interictal | |||||||
| Physiologic GE during sleep | Yes | Yes | Yes | Less visible on L hemisphere | Yes | Yes | Yes |
| Paroxystic GE | R F S and SW | R F-T S | BiT S and SW | No | No | Bilateral multifocal rare slow SW | BiF sharp waves |
| Slow waves | No | No | No | Yes/L hemisphere with F-C predominance | No | No | No |
| Sleep S activation | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Seizure recorded | No | No | No | No | No | No | No |
- BiF = Bi-frontal; C = central; EEG = encephalographic; F = frontal; GE = grapho-element; L = left; R = right; S = spike; SW = spike waves; T = temporal.
| Patient | II-2a | II-3a | V-1a | VI-1a | VII-1a | VIII-1a | VIII-2a |
|---|---|---|---|---|---|---|---|
| Awake interictal | |||||||
| Background activity | Normal (alpha) | Normal (alpha) | Normal (alpha) | Normal (alpha) | Slow (theta-alpha) | Normal (alpha) | Normal (alpha) |
| Spatial organization | Correct | Correct | Correct | Correct | Diffusing | Correct | Correct |
| Paroxystic GE | R F-T SW and rare sharp waves | R F-C-T SW | Rare R T sharp waves | R F-T SW and sharp waves | No | L T-O SW | No |
| Slow waves | No | No | No | No | No | Yes/L T-O | No |
| Photosensitivity | No | No | No | No | No | No | No |
| Sleep interictal | |||||||
| Physiologic GE during sleep | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Paroxystic GE | R F-T S | No | No | R F-T SW and sharp waves | No | No | No |
| Slow waves | No | No | No | No | No | No | No |
| Sleep S activation | Yes | No | No | Yes | No | No | No |
| Seizure recorded | No | No | No | No | No | No | No |
- C = central; EEG = encephalographic; F = frontal; GE = grapho-element; L = left; O = occipital; R = right; SW = spike waves; T = temporal.
The most commonly observed ictal symptoms were in descending order: orofacial automatisms in 70% (n = 7/10); upper limb dystonia or hypertonia in 50% (n = 5); apnea, cyanosis, or pallor in 40% (n = 4); eye blinking in 30% (n = 3); deviation of the head or the eyes in 30% (n = 3); a fall in 10% (n = 1); and hemi-body hypomobility in 10% (n = 1). Secondary generalization was observed in only one patient (II-1). Tachycardia was present in all recorded seizures except for one (III-1 in seizure 1), in which bradycardia was observed (heart rate below 60 beats per minute for around 30 seconds compared with heart rate at 115 beats per minute prior to seizure onset), and associated with autonomic alterations, such as pallor, cyanosis, and apnea.
Triggers for video-EEG recorded seizures were cutaneous contact with water (n = 7), defecating (n = 2), photosensitive (n = 2), emotional (n = 1), toothbrushing (n = 1), haircutting (n = 1), fingernail clipping (n = 1), and the idea of bathing (n = 1).
For all seizures recorded, the EEG showed at onset a rhythmic theta followed by delta activity predominating in temporal or temporo-perisylvian regions (n = 8/10; see Fig 2) or in frontal regions (2/10), diffusing to the ipsilateral hemisphere, and sometimes bilaterally. It then frequently evolved into a rhythmic spike-waves activity in the same regions. Postictal hemispheric (n = 4) or diffuse (n = 1) slowing activity could be observed. In one patient (I-1), a short, fast, low voltage activity in the right temporal region could be distinguished, evolving toward a rhythmic theta/delta activity. We provide an EEG sample (centered on the onset of the seizure) of one seizure from each patient in whom at least one seizure could be recorded by video-EEG (Supplementary Data).
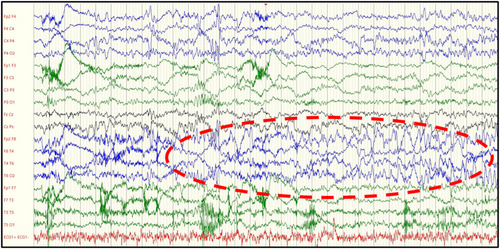
Overall, electro-clinical correlations showed that 80% (n = 8) of the seizures particularly involved the temporo-mesial or temporo-insular regions. For one patient (IV-1), the 2 seizures recorded evoked a frontal region onset. Seventy percent (n = 7) of the seizures originated in the right hemisphere and 30% (n = 3) in the left. Two out of 5 patients experienced multifocal seizures. Patient I-1 had almost clinically similar seizures but of the right and left temporal origin. The right and left frontal seizures were recorded in patient IV-1.
Interictal Descriptions
Interictal EEG analysis, both during wakefulness and sleep, were performed in all patients (n = 19; see Tables 4–7). The EEG background was normal in 17 patients (89%) and slow in 2 patients (11%). Slow waves were present in 4 patients, being focal in temporo-perisylvian regions in 3 and diffuse in 1 patient. No modification to photic stimulation or hyperpnea tests was identified. Physiologic sleep graphoelements were present and well organized for all patients, and less visible in only 1 patient.
Fifty-eight percent (n = 11) of the patients presented interictal paroxysmal graphoelements (spikes, spike-waves, or sharp waves). They were located mainly in the temporal (n = 4) or temporo-perisylvian regions (n = 6). In 1 patient, they were in the frontal region. A right hemispheric predominance was noted (n = 8). They were activated during sleep, becoming more frequent and sometimes spreading spatially to nearby regions. In 5 of the 11 patients with interictal paroxysmal graphoelements, they were rare (less than 1 per minute), so that 74% of the patients (n = 14/19) had rare or non-visible interictal paroxysmal graphoelements.
Video-EEG Psychogenic Non-Epileptic Seizures Description
PNES were recorded by video-EEG in 3 patients (16%) belonging to the same family (II-1, II-2, and II-3). The PNES could present clinical similarities with the semiology of associated epileptic seizures, albeit different. Patient II-1 had a PNES under the shower during video-EEG, presenting with atypical orofacial automatisms, impaired awareness, and a fall with no movement, no answer, and closed eyes, whereas the EEG did not record any abnormalities. Successive videos recorded in the same patient by his mother also showed very probable PNES with impaired consciousness, moderate hypersalivation, and agitation. Patient II-2 had 10 unprovoked PNES recorded by video-EEG with moderate disturbances of consciousness, forced eyes closure and blinking, head movements, and irregular/asynchronous generalized clonic movements. Patient II-3 had hypersalivation and chewing on video-EEG with a long duration and a possible memory recall of events during the episode, and no EEG modification. He also had numerous events at school with disturbed consciousness, blinking, and then closed eyes with no answer during several minutes.
Electroencephalographic Power Spectrum Analysis
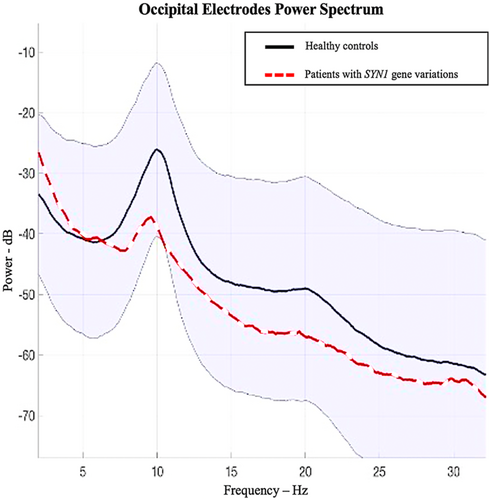
Nine patients with SYN1 pathogenic variations were excluded from the interictal connectivity and power spectrum analysis because some of their electrodes contained too many artifacts or were missing (n = 1, patient XV-1) so it was not possible to compare them to the healthy subjects, or because they were younger than 12 years old (n = 8). No major differences were observed between patients under and over the age of 12 years, in terms of epileptic characteristics or comorbidities (Fig 2).
For healthy subjects, a strong occipital alpha peak at 10 Hz was found, whereas patients with SYN1 variants (n = 10) had less power in the alpha band (Fig 3). A stronger predominance of the theta and delta frequency bands relatively to the alpha power-band in patients was also visually observed. These results are therefore in favor of a slower EEG rhythm in patients with SYN1 pathogenic variations.
Electroencephalographic Connectivity Analysis
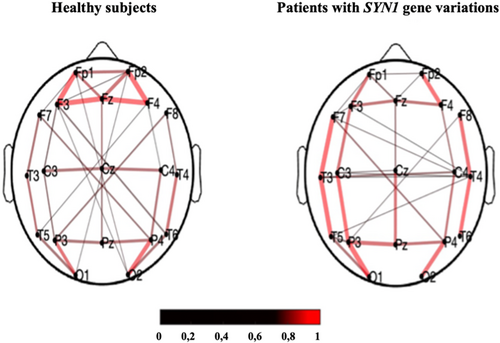
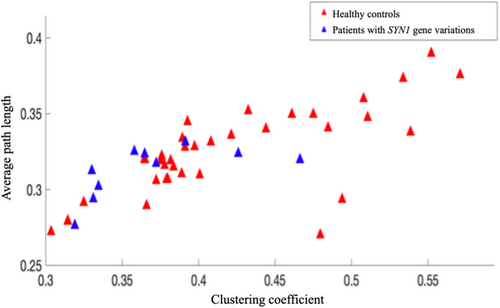
In healthy subjects, we observed a strong resting-state PLV connectivity in the frontal and occipital regions, with long distance connectivity links between these 2 regions identified. However, for patients with SYN1 variants (n = 10), the connectivity map was different, with the strongest connectivity links in both temporal regions (Fig 4). Two connectivity metrics were computed for each group: the clustering coefficient and the path length. Path length appeared to be similar, but a lower clustering coefficient was observed in patients with SYN1 variants compared to healthy control individuals (Fig 5).
Discussion
We analyzed video-EEG data of 10 epileptic seizures in 5 patients and interictal EEG data of 19 patients. To our knowledge, this is the largest reported cohort of patients with epilepsy related to SYN1 variants with detailed clinical and electrophysiological data. Based on the observation of shared clinical and EEG features among patients with SYN1-related epilepsy we suggest the delineation of a distinct epilepsy syndrome. Indeed, 80% of the seizures (8/10) recorded in video-EEG involved the temporal or temporo-insular/perisylvian regions. Ictal semiology consisted mostly of impaired awareness, automatisms, and autonomic symptoms. Ictal EEG showed a characteristic rhythmic theta/delta activity predominating in temporal or temporo-perisylvian regions at the beginning of most of the seizures. Only one patient (2 seizures) presented with electroclinical data evoking frontal lobe seizures.
To date, published data on electro-clinical patterns of seizures in patients with SYN1 pathogenic variations is rather scarce. A first publication in 20155 described 2 patients recorded with continuous video-EEG monitoring. The first patient was a 50-year-old man who presented 3 stereotypical seizures provoked by rubbing of his face with a wet cloth characterized by an aura of body chill, alteration of consciousness, manual automatisms, and rare secondary generalized tonic–clonic activity. Ictal EEG revealed an ictal right temporal rhythmic theta activity with subsequent propagation. The second patient was a 17-year-old boy who presented by bathing or nail clipping reflex seizures with focal temporal clinical onset. Ictal EEG disclosed rhythmic delta activity over the right temporal regions that quickly spread to parasagittal regions and evolved into a rhythmic spike and slow wave pattern. Sirsi et al25 reported 2 seizures recorded in video-EEG in a 7-year-old boy, triggered by contact with water. The electrographic seizure mainly evolved over the left temporal region. During the second seizure, ictal evolution was in the right fronto-temporal region. Ameen et al26 described a 6-year-old boy who presented water-reflex seizures with focal onset characterized by autonomic symptoms, mouth automatisms, and impaired awareness. Ictal EEG from an ambulatory home EEG, showed a rhythmic theta activity arising from the left fronto-temporal area. Peron et al10 reported focal seizures during contact with water in an 8-year-old patient. They were characterized by a sensation of rising heat, hemi-body dystonic posture, and cyanosis of the lips, followed by oral automatisms and inability to speak. Ictal EEG showed bilateral rhythmic theta activity over the fronto-central regions. Finally, Accogli et al15 reported 2 patients with bathing reflex autonomic seizures. Ictal EEG showed high-voltage polymorphic theta activity over the fronto-temporal areas.
All these case reports provide data which are rather consistent with our own, pointing to focal seizures originating or propagating rapidly to the temporal or temporo-insular/perisylvian regions. Autonomic ictal manifestations were frequently reported, in particular apnea, cyanosis or changes in cardiac rhythm, sometimes in the foreground. This finding also suggests involvement of temporal or temporo-perisylvian regions, in which cardiac changes are more frequently reported than in extra-temporal seizures.40 We found a right-hemisphere predominance in seizure organization, raising the question of possible hemispheric specialization. Indeed, some studies using intracerebral stimulations or neuroimaging have suggested that the right cerebral hemisphere is potentially more involved in sympathetic functions, and the left in parasympathetic functions, but more recent studies involving larger numbers of patients have not confirmed this hypothesis.41-44 Moreover, looking at the video-EEG cases reported in the literature, we do not find this preferential right-hemispheric lateralization of seizures. Of the 7 patients reported, 3 have seizures starting on the right hemisphere, 3 on the left, and 1 bilaterally.5, 10, 15, 25, 26 Thus, despite the right predominance of seizures’ organization in our data, the relatively small number of patients should make these data on lateralization taken with caution and further studies, including a larger numbers of patients, should clarify this point.
Moreover, the high prevalence of oro-facial automatisms at the onset of the recorded video-EEG seizures suggests likewise the involvement of temporo-mesial and insular regions in the genesis of these seizures.45, 46
Interictal paroxysmal graphoelements (such as spikes or spike-waves) were rare or absent in almost 70% of our patients (n = 13/19), and, when present, tended to be localized in the temporo-perisylvian regions. This is compatible or even suggestive of a deep seizure onset, possibly in the temporal or insular regions. Similarly, an ictal EEG pattern was also evocative, with rhythmic theta activity at the onset of most seizures, followed by delta activity predominating in the temporal or temporo-perisylvian regions, diffusing into the ipsilateral hemisphere, and sometimes bilaterally. This pattern also reported in the few patients with SYN1-related epilepsy who benefited from a video-EEG recording,5, 15, 25 is also consistent with a deeply localized seizure onset, having similarities with what has been reported in mesial temporal seizures.47, 48
This electroclinical pattern of focal seizures, involving mainly the temporo-insulo/perisylvian regions, is somewhat different from what one could expect when based on seizure descriptions reported by caregivers and parents. Although focal seizures are mentioned, seizures with prominent motor manifestations or seizures with a generalized appearance are frequently reported,21 probably because they are easier to identify. It is likely that some manifestations of focal seizures go unnoticed because they are either subtle, or can only be reported subjectively, which is impossible in young children or patients who cannot verbally express their sensations. This highlights the importance of video-EEG recordings to gather accurate electro-clinical information to support the choice of the most appropriate antiseizure medications and to provide more data about the pathogenesis of this epilepsy syndrome.
Moreover, we recorded PNES on video-EEG in 3 of our patients. It is likely that the frequency of PNES in patients with SYN1-related epilepsy remains underestimated. Especially if, as in our patients, some PNES are triggered by cutaneous contact with water, also having clinical similarities with the truly epileptic paroxysmal events. Video-EEG recordings are therefore also important for identifying PNES to avoid unnecessary modifications in antiseizure medications. Genetic variants associated to neurologic disorders may be a potential hypothesis to explain the high frequency of PNES occurring in this epilepsy syndrome.49
We observed a more important proportion of ID in the patients with an epileptic seizure onset before the age of 5 years, but we did not find a link between the severity of epilepsy and cognitive/behavioral impairment. It suggests that cognitive impairment processes are not the sole consequence of epileptic seizures but are most likely also related to parallel pathophysiological processes resulting from the SYN1 gene alteration. Similarly, Parenti et al (2022)21 did not detect enrichment in developmental delay, ID, or ASD in patients with epilepsy compared with non-epileptic patients, although they noted an association between a younger age at seizure onset and the severity of ID, and the loss of acquired milestones for some individuals after the onset of seizures. Neurological features of some patients, but not all, with SYN1 variants seem therefore to correspond to a developmental and epileptic encephalopathy, as a part of developmental difficulties seem to be arising directly from the effect of the genetic mutation, with an additional effect of the epileptic activity on development.50 The shift toward slower frequency bands of interictal EEG observed in patients with SYN1 variants compared to healthy subjects has also been described in neurodevelopmental disorders, such as ADHD.51, 52 However, no statistical analysis could be performed in our study because of the small group size.
Epilepsy usually begins in childhood and seizures often persist through adult life, with possible drug-resistance. A very particular clinical feature is the appearance of reflex seizures, which, in our series, occurred in 63% of patients. This appears to be in line with previously published data, as several triggers have been reported in individual patients with SYN1 variants, initially and mainly water-related, such as showering or bathing, but also rubbing with a towel, cutting hair, clipping nails, the thought of taking a bath, brushing teeth, defecating, and laughing.15, 20, 21, 25, 26 Reflex epilepsy can be defined as the presence of recurrent seizures in response to a specific motor, sensory, or cognitive stimulation.53-55 Exact pathophysiological mechanisms remain unknown but probably include a hyper-excitability of cortical or subcortical neuronal areas in response to a physiologic stimulus. It is noteworthy that most of the triggers reported in SYN1-related epilepsy correspond to somatosensory stimuli. Most patients have a single reproducible trigger for their seizures. Interestingly, some patients showed a more heterogeneous sensitivity to various triggers (such as cutaneous contact with water and toothbrushing; or cutaneous contact with water, haircutting, and fingernail clipping), raising the question of possibly different pathophysiologic mechanisms. However, it is noticeable that (except for 2 patients, who had a visual trigger), all the triggers are somato-sensory and body-centered and so probably involve the same somato-sensory networks. This is consistent with the description of temporo-insular seizures we report in our patients, as the insula is one of the most important brain areas for the processing of somatosensory and thermal stimuli, particularly in its medium/posterior part.56 Somatosensory physiological stimuli, such as cutaneous contact with water, could thus trigger seizures by activating genetically based hyperexcitable temporo-insular networks.
This is consistent with the increased connectivity in both temporo-perisylvian regions we visually found in patients with SYN1 variants compared with healthy subjects. Our findings suggest a reorganization of networks, and could support a hyperexcitability of these regions, perhaps at the origin of the onset of seizures in these patients.27, 29, 30, 32
Our study comprehensively defined the electroclinical features of epilepsy in patients with SYN1 pathogenic variants. A distinct epilepsy syndrome emerged, the phenotype of which can be described as follows: (1) EEG background and organization are mainly normal or slightly slow, in wakefulness and sleep; (2) interictal abnormalities are often rare or not visible on EEG; when visible, they mostly consist of spikes or spike-waves in temporal or temporo-perisylvian regions; (3) more than 60% of patients have reflex seizures (cutaneous contact with water and defecation being the main triggers) isolated or associated with spontaneous seizures; (4) electro-clinical semiology of seizures is mainly temporal or temporo-insulo/perisylvian with a notable autonomic component; and (5) ictal EEG shows a characteristic rhythmic theta/delta activity predominating in temporal or temporo-perisylvian regions at the beginning of most seizures. The association of the above in features in children with (or without) neurodevelopmental or behavioral difficulties should help to guide genetic diagnosis and facilitate the identification of new families sharing the same genetic condition.
Acknowledgments
The authors thank the networks ERN EpiCARE (European Reference Network for rare and complex epilepsies) and LFCE (French League Against Epilepsy) by announcements, for increasing the number of patients included in this study, and all individuals, including patients and their families, who participated in the study, as well as the clinicians, researchers, and nursing staff of all institutions that contributed to the investigations.
Author Contributions
V.M.Q., A.Ad., and L.M. contributed to the conception and design of the study. V.M.Q., A.Ad., H.S., A.Ar., P.Co., G.L., F.M., A.B., H.B., J.P.C., P.Ca., L.C., Y.C., M.C., C.D., V.De., J.D.R., V.Di., E.G., G.J.K., J.J., M.L.M., M.M.M., M.M., A.L.P., K.P., A.R., M.F.S., A.T.V.S., G.W., and L.M. contributed to the acquisition and analysis of data. V.M.Q., A.Ad., and L.M. contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest
Nothing to report.
Open Research
Data Availability
The data that support the findings of this study are available on request from the corresponding authors V.M.Q. and L.M.