GGPS1 Mutations Cause Muscular Dystrophy/Hearing Loss/Ovarian Insufficiency Syndrome
Abstract
Objective
A hitherto undescribed phenotype of early onset muscular dystrophy associated with sensorineural hearing loss and primary ovarian insufficiency was initially identified in 2 siblings and in subsequent patients with a similar constellation of findings. The goal of this study was to understand the genetic and molecular etiology of this condition.
Methods
We applied whole exome sequencing (WES) superimposed on shared haplotype regions to identify the initial biallelic variants in GGPS1 followed by GGPS1 Sanger sequencing or WES in 5 additional families with the same phenotype. Molecular modeling, biochemical analysis, laser membrane injury assay, and the generation of a Y259C knock-in mouse were done.
Results
A total of 11 patients in 6 families carrying 5 different biallelic pathogenic variants in specific domains of GGPS1 were identified. GGPS1 encodes geranylgeranyl diphosphate synthase in the mevalonate/isoprenoid pathway, which catalyzes the synthesis of geranylgeranyl pyrophosphate, the lipid precursor of geranylgeranylated proteins including small guanosine triphosphatases. In addition to proximal weakness, all but one patient presented with congenital sensorineural hearing loss, and all postpubertal females had primary ovarian insufficiency. Muscle histology was dystrophic, with ultrastructural evidence of autophagic material and large mitochondria in the most severe cases. There was delayed membrane healing after laser injury in patient-derived myogenic cells, and a knock-in mouse of one of the mutations (Y259C) resulted in prenatal lethality.
Interpretation
The identification of specific GGPS1 mutations defines the cause of a unique form of muscular dystrophy with hearing loss and ovarian insufficiency and points to a novel pathway for this clinical constellation. ANN NEUROL 2020;88:332–347.
The muscular dystrophies are degenerative disorders of muscle characterized by evidence of degeneration and regeneration on muscle histology and are typically associated with elevated creatine kinase (CK) as a measure of ongoing muscle fiber breakdown. Mechanisms and pathways that underlie muscular dystrophies are diverse and include extracellular, membrane-centered, sarcomeric, nuclear, and mitochondrial as well as metabolic mechanisms.1 In the dystrophies that involve membrane stability or repair, serum CK values are typically elevated as a sign of membrane leakage. Congenital sensorineural hearing loss rarely manifests in association with muscular dystrophy but has been reported in early onset facioscapulohumeral muscular dystrophy.2 Perrault syndrome, caused by mutations in various mitochondrial and peroxisomal genes, clinically manifests with sensorineural hearing loss and primary ovarian insufficiency but without muscle involvement3, while a combination of muscular dystrophy with primary ovarian insufficiency has not been recognized yet.
The mevalonate pathway is a central metabolic shuttle for the synthesis of specialized lipids. Mevalonic acid is formed from acetyl-CoA via HMG-CoA and then further metabolized to isopentenyl pyrophosphate (IPP), which is subsequently converted to farnesyl pyrophosphate (FPP), which serves as a precursor for the squalene cholesterol pathway as well as for protein prenylation,4 which includes farnesylation as well as geranylgeranylation. Prenylation adds a hydrophobic lipid tail to proteins, allowing for anchorage to membranes for precise subcellular localization, typically within membranes. Prenylation is of particular relevance for small guanosine triphosphatases (GTPases), including those of the Rho and Ras/Rab families. For geranylgeranylation, FPP has to be converted to geranylgeranyl pyrophosphate (GGPP), by geranylgeranyl diphosphate synthase (GGPPS; EC:2.5.1.29), encoded by GGPS1. GGPP will then be transferred by 2 types of geranylgeranyl transferase (GGTase I or GGTase II) onto the recipient proteins. Whereas some of the small GTPases are only farnesylated or can alternatively be farnesylated or geranylgeranylated, some are exclusively dependent on geranylgeranylation for their prenylation. More than 100 human proteins are predicted to be targets for geranylgeranylation; among them are prominently small GTPases: such as the Rab family, many of which are involved in tagging membranes of endoplasmic reticulum, Golgi, endosomes, lysosomes, autophagic vesicles, cell membranes, and mitochondria, as well as of the Rho/Rac family, which are involved in cytoskeletal actin dynamics among other roles.5-7 Other functions of GGPP include negative feedback regulation of the mevalonate pathway by inducing HMG-CoA reductase misfolding, ubiquitination, and degradation.8 Some GGPP may also enter into ubiquinone, coenzyme Q, and squalene synthesis; however, FPP is the major source for this biosynthetic branch. It is also possible that there are additional, not yet explored functions and uses for GGPP in the cell.9 Although thus far there has been no human monogenic disease linked to mutations in GGPS1, it has been identified as a risk factor for atypical femoral bone fractures in females exposed to bisphosphonates.10-12
Here, we report specific mutations in the gene GGPS1 coding for the enzyme geranylgeranyl diphosphate synthase in 11 patients from 6 families, causing a highly distinctive syndrome of early onset muscular dystrophy combined with congenital sensorineural hearing loss and primary ovarian insufficiency in females. The mutations do not abolish enzymatic activity of this essential enzyme, but with a highly distinctive genotype/phenotype correlation define the mevalonate pathway as essential for muscle, hearing, and endocrine functions.
Subjects and Methods
Patient Recruitment and Sample Collection
Patients P1, P2, and P7 were consented and enrolled in the study approved by the institutional review board (IRB) of the National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH; 12-N-0095). Patient P3 was enrolled via the Institute of Genetic Medicine, International Centre for Life's Biobank (National Research Ethics Service Committee North East–Newcastle & North Tyneside; Research Ethics Committee [REC] reference: 08/H0906/28+5). Patients P4–P6 and patient P8 were enrolled via the Institute of Myology and Public Hospital Network of Paris, Pitié-Salpêtrière Hospital Group (REC reference: AC-2018-3156). Patients P9–P11 were consented and enrolled to the MYOGENE project, approved by the Institute of Genetics and Molecular and Cellular Biology IRB (DC-2012-1693). Tissue (DNA and skin fibroblasts) were obtained based on standard procedures. Muscle biopsies were obtained as part of the regular clinical diagnostic testing and were evaluated by standard light and electron microscopy protocols.
Molecular Genetic Analyses of Patients
Whole exome sequencing (WES) was performed in the 2 index patients (Patients P1 and P2) at the NIH Intramural Sequencing Center using the Illumina (San Diego, CA) TruSeq Exome Enrichment Kit and Illumina HiSeq 2500 sequencing instruments, and was combined with haplotype analysis in the entire family (as described13). Assuming autosomal recessive inheritance in a nonconsanguineous family, the haplotype data were analyzed to identify regions of haplotypes shared between the 2 affected siblings but not their unaffected siblings or parents. WES was performed in the 2 affected siblings (Patients P1 and P2) and analyzed for biallelic rare variants that were predicted to be damaging and shared between them. Given the highly specific-appearing clinical phenotype in the index family, an additional 6 patients (Patients P3–P8) in 4 families were identified at neuromuscular centers in Europe and Asia. DNA was analyzed by direct Sanger sequencing of the candidate gene. In one additional family, mutations in the candidate gene were identified in 3 affected siblings (Patients P9–P11) via WES, which was performed using the SureSelect Human All Exon 50 Mb capture library v5 (Agilent Technologies, Santa Clara, CA) and the Illumina HiSeq2500 platform. Sequence data were aligned to the GRCh37/hg19 reference genome, variants were annotated and filtered based on their frequency in Genome Aggregation Database (gnomAD; http://gnomad.broadinstitute.org/) and an in-house database containing >1,500 exomes, and the impact of the variants was predicted using SnpEff (http://snpeff.sourceforge.net/).
Histology and Immunohistochemical Staining
Muscle biopsies and skin biopsies were processed following standard procedures. Dermal fibroblasts derived from skin biopsies were cultured as described previously.14 Some of the dermal fibroblasts were converted to myoblasts with the Lenti-MyoD lentiviral system (Clontech Laboratories, Mountain View, CA). Cultured fibroblasts and muscle cryosections were processed with a standard immunohistochemical staining method.14 The following antibodies were used for immunohistochemical staining: GGPS1 (N-term; Abcepta, San Diego, CA), desmin (clone DE-U-10; Sigma-Aldrich, St Louis, MO), LC3b (D11; Cell Signaling Technology, Beverly, MA), and ATP synthase beta (Thermo Fisher Scientific, Waltham, MA). Confocal images were captured with a Leica (Wetzlar, Germany) SP5 confocal laser scanning microscope.
GGPPS Enzyme Activity Assay and Conformation Analysis
Wild-type GGPPS and mutant GGPPS identified in the patients were expressed in a bacterial expression system; enzyme activity was assayed using the substrates farnesyl pyrophosphate (FPP) and C14-isopentenyl pyrophosphate (C14-IPP) as described previously.12 The GGPPS activities in Lenti-MyoD–converted myoblasts of normal controls and patients were also assayed as described before.15 The oligomerization of wild-type and mutant GGPPS expressed in a bacterial system was measured by gel filtration chromatography using an ÄKTA Explorer as described previously.12
Generation of a Ggps1 p.Y259C Knock-In Mouse
A knock-in mouse was generated under contract at genOway (Lyon, France) by insertion of a Y259C point mutation in exon 5a of the Ggps1 gene (located 633bp downstream of the 5′ end of exon 5a). The mutated Ggps1 gene is expressed under the control of the endogenous Ggps1 promoter. In addition, the Ggps1 exon 4 is flanked by a validated flippase recognition target (FRT)-neomycin-FRT-loxP cassette and by a single loxP site enabling the conditional deletion of the Ggps1 gene. The FRT-neomycin-FRT-loxP cassette and the single loxP site are positioned as illustrated in the final figure.
Cell Membrane Repair Assay
Dermal fibroblasts derived from normal controls and Patient P6 were MyoD transfected using Lenti-MyoD, converted to myoblasts, and further differentiated into myotubes. These cells were tested in separate experiments for their cell membrane repair kinetics following focal pulsed laser injury in the presence of the cell impermanent dye FM 1-43 and following the dye intensity as described.16
Results
Clinical Phenotype
The index patient (Patient P1; Fig 1) manifested symptoms prenatally with decreased fetal movements, and neonatally with a weak cry and a poor suck. By 12 months of age, she was diagnosed with bilateral sensorineural hearing loss (via brainstem auditory evoked response testing). She had delayed early motor milestones and walked independently at 18 months of age. She had intermittent episodes of diarrhea accompanied by poor feeding, muscle weakness, and elevated CK (up to 18,025U/l at 19 months of age). Progressive muscle weakness and contractures resulted in full-time wheelchair use by 11 years of age. She developed respiratory insufficiency, necessitating noninvasive ventilation, progressing to tracheostomy. Progressive scoliosis necessitated spinal fusion at age 11 years. At 14 years of age, she had a gastrostomy tube placed for failure to thrive. She had amenorrhea, and following an endocrinologic evaluation was diagnosed with primary ovarian insufficiency. The index patient's younger brother (Patient P2) presented with a similar phenotype (Table), with congenital sensorineural hearing loss, muscular dystrophy with intermittent high elevations in CK (up to 11,250U/l), respiratory insufficiency, and scoliosis. His fertility remains unknown, and he has not undergone a formal endocrinologic evaluation. Whole exome sequencing was performed and was superimposed on shared haplotype regions, resulting in the identification in Patients P1 and P2 of biallelic variants (Y259C; R261G) in the gene GGPS1 coding for the enzyme geranylgeranyl diphosphate synthase.

| Patient Identifier | Family 1 | Family 2 | Family 3 | Family 4 | Family 5 | Family 6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | |
| GGPS1 mutationsa | p.[Tyr259Cys; Arg261Gly] c.[860A > G; 865C > G] | p.[Pro15Ser; Arg261Gly] c.[127C > T; 865C > G] | Homozygous p.Arg261His c.866G > A | Homozygous p.Arg261His c.866G > A | Homozygous p.Phe257Cys c.854 T > G | Homozygous p.Phe257Cys c.854 T > G | |||||
| Sex | F | M | M | F | M | F | F | M | M | F | F |
| Ethnicity | Irish, German, and Polish | English | Moroccan | Chinese (Han) | Algerian | Moroccan | |||||
| Current age, yr | 31 | 29 | 22 | 46 | 45 | 44 | 14 | 21 | 22 | 11 | 8 |
| Age at last evaluation, yr | 28 | 26 | 21 | 44 | 42 | 43 | 14 | 14 | 18 | 7 | 4 |
| Sensorineural hearing loss | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Wheelchair dependence (age) | Y (11 yr) | Y (13 yr) | Y (15 yr) | N | N | N | N | NA | Y (12 yr) | Y (11 yr) | N |
| Respiratory insufficiency | Y | Y | Y | Y | Y | Y | Y | NA | Y | N | N |
| FVC, % predicted | NA | NA | NA | 74% | 67% | 79% | 47% | NA | NA | NA | NA |
| Scoliosis | Y | Y | Y | N | N | N | N | NA | Y | N | N |
| Maximal recorded CK, U/l | 18,025 | 11,250 | 6,641 | 336 | 4,900 | 281 | 14,143 | 1,600 | 3,633 | NA | NA |
| Primary ovarian failure | Y | — | — | Y | — | Y | Uncertain due to age | — | — | Uncertain due to age | Uncertain due to age |
| Failure to thrive | Y | Y | Y | N | Y | N | Y | NA | Y | Y | Y |
- a = [NP_001032354.1] [NM_001037277.1] CK = creatine kinase; F = female; FVC = forced vital capacity; M = male; N = no; NA = not available; Y = yes; yr = years.
A further 9 patients in 5 families with recessively inherited mutations in GGPS1 were subsequently identified, using direct GGPS1 sequencing based on a suggestive phenotype (Patients P3–P8) or analysis of WES results (Patients P9–P11; see Table). Patient P3 was born prematurely (29 weeks gestational age) and was diagnosed with congenital sensorineural hearing loss. He carries compound heterozygous mutations in GGPS1 (P15S; R261G) and has a phenotype most similar to Patients P1 and P2, with whom he shares the R261G mutation. Like Patients P1 and P2, Patient P3 has had variably elevated CK values (up to 6,641U/l) and has developed respiratory insufficiency, wheelchair dependence, scoliosis, and failure to thrive. In Family 3, 3 of 6 children (Patients P4-P6) are affected and carry homozygous R261H mutations in GGPS1, manifesting with a milder motor phenotype in that they all have muscle weakness but achieved the ability for a slow run and have maintained independent ambulation into adulthood. All have sensorineural hearing loss, and both affected females have been formally diagnosed with primary ovarian insufficiency. The male has not undergone a formal endocrinologic evaluation but has no offspring at 45 years of age. Patient P7 in Family 4 is also homozygous for the R261H mutation in GGPS1 and also manifests a comparatively milder phenotype from a motor perspective. At 14½ years of age, she remains independently ambulatory. Patient P8 in Family 5 is homozygous for the F257C mutation in GGPS1 and was diagnosed with congenital sensorineural hearing loss. At the time of his last assessment at 14 years of age, he remained independently ambulatory. In Family 6, 3 of 5 children (Patients P9–P11) were identified via WES to carry homozygous F257C mutations in GGPS1, resulting in a moderate-to-severe phenotype, with all 3 siblings manifesting muscle weakness and failure to thrive. The oldest sibling (Patient P9, a 22-year-old male) has been a full-time wheelchair user since the age of 12 years, and he has respiratory insufficiency and scoliosis. Of the younger (female) siblings, the 11-year-old (Patient P10) uses a wheelchair full-time, while the 8-year-old (Patient P11) remains independently ambulatory.
Thus, progressive muscle weakness and joint contractures resulting in loss of independent ambulation, respiratory insufficiency necessitating ventilation dependence, and severe scoliosis were seen in Patients P1, P2, P3, and P9, whereas the other patients had a less severe progression of weakness. Sensorineural hearing loss was present in all but one patient in this cohort and was the first symptom identified in those patients with milder motor phenotypes. Given that the hearing loss was diagnosed in some patients in the neonatal period and in others during childhood, the onset is likely congenital, as diagnosis relies on a formal audiometric evaluation. Primary ovarian insufficiency, also known as premature ovarian failure, has been diagnosed in all postpubertal females in this cohort, as confirmed by menopausal levels of follicle-stimulating hormone (FSH) in the setting of a history of amenorrhea (Patient P1, FSH: 88.2IU/l) or infertility (Patient P4, FSH: 50.3IU/l; Patient P6, FSH: 53.2IU/l). Eight patients had evidence of failure to thrive and/or short stature, potentially suggestive of further endocrinologic involvement. Of note, no cardiac or cognitive involvement has been noted in this cohort.
Whole-body muscle magnetic resonance imaging was performed in Patients P4–P6 and demonstrates variable increase in T1 signal in select muscles, resulting from fatty infiltration and thus consistent with an underlying muscular dystrophy (see Fig 1C). It is notable that these 3 patients all demonstrated relative sparing of the rectus femoris, sartorius, and gracilis muscles. Clear asymmetry of muscle involvement and heterogeneously distributed abnormal T1 signal in the hamstrings were seen in Patients P5 and P6.
Muscle biopsies were performed in 9 patients (Patients P1–P9). Histology was dystrophic, with evidence of degeneration and regeneration and internalized nuclei. There were occasional rimmed vacuoles; the oxidative stains had peripherally increased oxidative reactivity in some fibers and core-like regions in other fibers. Electron microscopy revealed ultrastructural evidence of enlarged but structurally normal mitochondria (Patient P8) as well as evidence of excess autophagic material (Patients P1 and P8; Fig 2). The CoQ10 level in muscle was determined to be normal in Patient P1. Of note, Patient P3 was given an empirical treatment trial of oral CoQ10, which did not result in any noted improvement of symptoms.
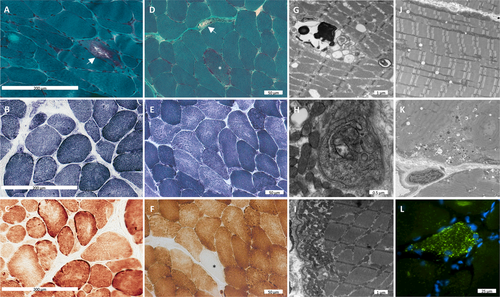
In summary, GGPS1-related muscular dystrophy/hearing loss/ovarian insufficiency syndrome is characterized by a remarkably consistent and recognizable phenotype of congenital sensorineural hearing loss, primary ovarian insufficiency in females, proximal muscle weakness with episodes of variably elevated CK, scoliosis, and respiratory insufficiency, in which the symptoms are progressive in nature and can be severe. Muscle histological and ultrastructural findings are consistent with a dystrophic process with evidence of abnormal autophagy and mild mitochondrial changes.
Gene Identification and Mutations
In the index family, WES in the 2 index patients (Patients P1 and P2) was combined with haplotype analysis on the entire family, which ruled out 97.3% of the exome, leaving 2.07% consistent with recessive compound heterozygous inheritance. The compatible region was distributed on 3 separate regions on chromosome 1, and 1 region on chromosome 3, covering 55,538,583bp and containing 745 genes. Subsequently, WES data in P1 and P2 were analyzed for shared biallelic rare variants that were predicted to be damaging. Two variants (Y259C and R261G) in the geranylgeranyl diphosphate synthase 1 or GGPS1 gene located in one of the compatible regions on chromosome 1 were identified as the only likely candidates to be responsible for the phenotype in this family. Both were extremely rare; Y259C was not present in gnomAD (https://www.biorxiv.org/content/10.1101/531210v3), whereas R261G was listed once, and both were predicted to be damaging (PolyPhen-2 prediction score of 1.000 and 0.711 for Y259C and R261G, respectively). Mutation and segregation in the family were confirmed by Sanger sequencing.
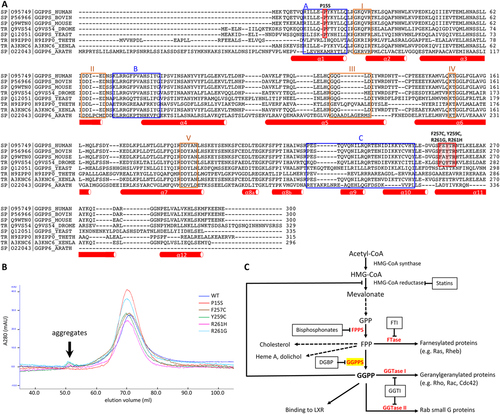
Given the highly specific-appearing clinical phenotype in the index family, an additional 6 patients in 4 families were identified at neuromuscular centers in Europe and Asia and analyzed by direct sequencing of GGPS1. One additional family (Family 6) with 3 affected siblings was identified by WES. In all patients, we identified biallelic pathogenic variants in GGPS1; 4 families were found to be homozygous, and in the remaining family compound heterozygous variants were identified. In all families, missense variants were identified: F257C (2 families), R261H (2 families), and R261G in compound heterozygosity with P15S in Family 2 (Patient P3) and Y259C in Family 1 (Patients P1 and P2). Allele frequencies (AFs) as determined in gnomAD were very low: P15S, AF = 0; F257C, AF = 0; Y259C, AF = 0; R261G, AF = 0.00003; R261H, AF = 0.00002. Rare heterozygous carriers for loss-of-function variants in GGPS1 are listed in gnomAD and should therefore be asymptomatic. The pathogenic variants fell within a specific 5 amino acid region toward the C terminus around the start of helix 11, with as of yet unclear function (Fig 3A). In animals, geranylgeranyl diphosphate synthase is formed as a hexamer and in certain circumstances as an octamer, whereas in plants it is a dimer.17 In silico modeling of GGPPS hexamer shows that this domain, in which all but one of the pathogenic variants (P15S) were clustered, is located toward the outside of the hexamer and not in the barrel where the catalytic core of the enzyme is located (Fig 4A). The first α-helix containing the P15S mutation is conserved and involved in the formation of the trimer from the dimers (see Fig 3A). While the catalytic domain itself is highly conserved across plants and animals, the mutated 5 amino acid domain is highly conserved in animals but not in plant ggps1, suggesting a function beyond the basic enzymatic activity of the enzyme in animal cells.
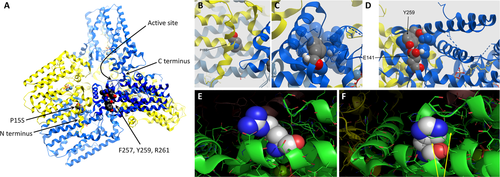
We were unable to generate a crystal structure of mutant GGPPS, while in silico modeling of the pathogenic variants did not result in major conformational or charge distribution changes of the 11th α-helix (see Fig 4B–D); only R261G results in the loss of a positive charge (see Fig 4E, F).
Immunohistochemical Analysis
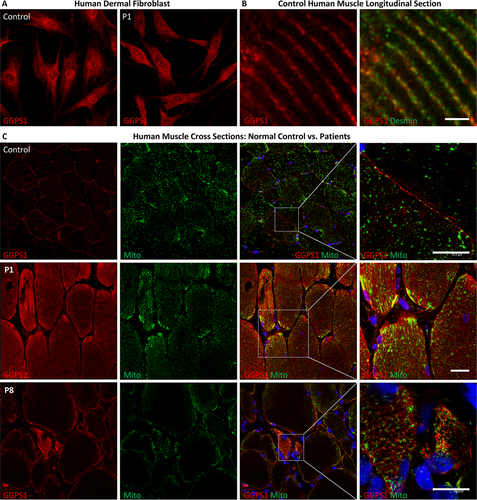
In primary human dermal fibroblast, GGPPS immunocytochemistry revealed a rather uniform cytoplasmic expression consistent with its known cytosolic localization,18 along with signals concentrated at some organelles and the perinuclear region (Fig 5A). There was no obvious difference in this pattern in patients, as seen in Patient P1 compared to controls. In longitudinal sections of control human muscle, GGPPS immunohistochemistry showed prominent localization to the Z disc, which was labeled with desmin (Fig 5B). In patients, there was no deficiency of GGPPS immunoreactivity compared to controls; it appeared brighter and showed some focal, subsarcolemmal accumulation, partially colocalizing with the mitochondrial marker adenosine triphosphate (ATP) synthase beta (Fig 5C).
Enzymatic Activity
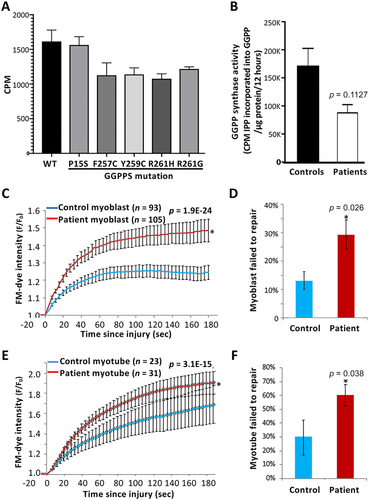
We first measured enzyme activity of wild-type and mutant GGPPS expressed in bacteria. Using 10μM farnesyl pyrophosphate (FPP)/C14-isopentenyl pyrophosphate (C14-IPP) as a substrate showed that the P15S mutant had the same activity as wild-type; however, the other mutants (F257C, Y259C, R261G, and R261H) consistently showed a reduced rate of activity of approximately 70 to 85% of wild-type (Fig 6A). Activity assays performed with 20μM FPP/C14-IPP in the presence of 20μM GGPP (thus a concentration of GGPP just below the concentration required for product inhibition in the wild-type enzyme) also had no effect on the enzyme activity in the mutants, suggesting that the mutations were not causing abnormal product release or an increase in their sensitivity to product inhibition (data not shown). Next, we measured GGPPS enzymatic activity in Lenti-MyoD–converted myoblasts of normal controls and patients (see Fig 6B). We found that enzymatic activity in samples pooled from 4 patients (Patients P1, P2, P6, and P8) was decreased to about 50% of the activity in a pooled normal control sample.
GGPPS Conformation
GGPPS has been shown to be hexameric in structure as a trimer of dimers.15 The size of the enzyme produced in the bacterial expression system in solution was measured using gel filtration chromatography. We found that mutant enzymes eluted essentially at a similar point to the wild-type enzyme (see Fig 3B). The molecular weight of each mutant enzyme was in the expected range of 240 to 255kDa, suggesting the complexes are hexameric. There was a small amount of aggregated protein apparent for mutants Y259C, R261H, and R261G, which eluted with the void volume, but no indication of any smaller enzyme unit such as the dimer.
Cell Membrane Repair following Focal Injury
Patient fibroblasts were used to generate myoblasts, which were then differentiated into myotubes prior to testing the cell membrane repair kinetics and the ability of these cells to repair from focal laser injury as described.16 We observed greater dye entry, indicating a poor cell membrane repair, in both the patient myoblasts and myotubes as compared to the healthy controls (see Fig 6C, E traces). Consequently, there was about a 2-fold increase in the number of patient myoblasts that failed to repair (see Fig 6D). Interestingly, despite the experimental challenge associated with the greater fragility of myotubes grown on a dish, which results in many more myotubes (compared to myoblasts) detaching upon injury, the patient myotubes demonstrated a 2-fold reduction in their ability to survive focal injury as compared to the control myotubes (see Fig 6F).
Ggps1 p.Y259C Knock-In Mouse
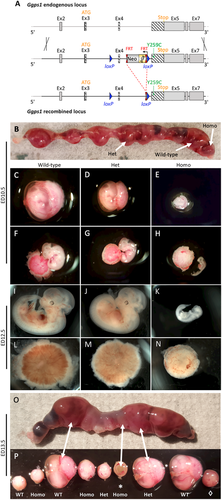
Given that total enzymatic activity was only moderately decreased in vitro, and that the identified mutations cluster in a very narrow, externally facing domain outside of the catalytic core of the enzyme, we surmised that the human disease-associated mutations would likely only interfere with a highly specific function or subcellular localization of the protein to explain the very specific clinical phenotype. As a total knock-out the gene is not viable; we therefore created a knock-in mouse of the mutation p.Y259C (Fig 7A) detected in compound heterozygosity in the index family (Family 1) to study the effects of the mutant GGPS1 on the organismal level. We chose this mutation, with the highest PolyPhen-2 score, in order to maximize our chances of detecting a phenotype in the mouse, as knock-in models of human missense mutations are frequently insufficiently penetrant. We were surprised to find that no litter included homozygous mutant animals. Timed pregnancies (see Fig 7B–P) revealed that homozygote Y259C knock-in embryos developed up to embryonic day (ED)12.5, with no live embryos observed after this point. No gross developmental abnormalities but a dramatically slowed rate of development was evident at ED10.5, when homozygous Y259C knock-in embryos more closely resembled a wild-type embryo at ED9.5,while at ED12.5 they resembled the typical development observed in wild-type embryos at ED10. Thus, detailed mechanistic studies relevant to the human phenotype were not possible in this mouse strain.
Discussion
There are 3 important clinical dimensions to this recognizable syndrome: muscular dystrophy, hearing loss, and infertility. The muscular dystrophy ranged from prenatal/congenital onset with progression to loss of ambulation and respiratory failure in early teenage years, to milder manifestations with preserved ambulation into adulthood (see Table). In Patients P1, P2, and P7, there was a history of episodic worsening of weakness concomitant with higher CK elevations during episodes of diarrhea. Muscle magnetic resonance imaging performed in Family 3 (see Fig 1C) revealed variable evidence of fatty replacement of muscles consistent with a muscular dystrophy. The relative sparing of the rectus femoris, sartorius, and gracilis muscles and asymmetric involvement of muscles in this clinical context may be diagnostically helpful but can also be observed in other muscular dystrophies. Imaging of additional patients will be required to assess whether a consistent pattern will emerge. Muscle histology findings confirm degenerative features of muscular dystrophy but with ultrastructural findings of disordered autophagy and larger, but structurally normal-appearing, mitochondria, pointing to potential involvement of these organelles (see Fig 2).
To our knowledge, this triad of clinical findings has not been seen in another form of muscular disease. Notably, the sensorineural hearing loss (confirmed in all but one patient in this cohort) was congenital in onset, as it was apparent as a delay in early language acquisition or evident on the first formal audiometric testing performed. Primary ovarian insufficiency was present in all postpubertal females in this cohort, as confirmed by abnormally high FSH levels when evaluated for amenorrhea (Patient P1) or infertility (Patients P4 and P6). The manifestations of sensorineural hearing loss and primary ovarian insufficiency were not related to the severity of the muscular dystrophy. Although we were not able to obtain andrological examinations on the postpubertal male patients in our series, none of the 5 male adult patients has had children. Of interest, reduced expression of GGPPS in testis has been linked to infertility in men.19, 20
The sensorineural hearing loss observed in patients with GGPS1-related muscular dystrophy is most likely sensory organ–related and localized to the organ of Corti, consistent with cochlear implantation being highly effective in all 4 patients in our cohort who underwent implantation. Hereditary sensorineural hearing loss is genetically and mechanistically very heterogeneous.21, 22 None of the recognized forms of hereditary sensorineural hearing loss has muscular dystrophy associated as part of the phenotype. Hearing loss may occur in combination with a muscular dystrophy or a myopathy in early onset facioscapulohumeral dystrophy2, 23 and Vici syndrome, a complex congenital multisystemic disorder caused by recessive mutations in autophagy regulator EPG5 gene, which can also be associated with sensorineural hearing loss,24-27 as well with a vacuolar myopathy,28 thus implicating abnormal autophagy regulation in the causation of sensorineural hearing loss and myopathy. Early onset sensorineural hearing loss and primary ovarian insufficiency without muscular dystrophy have been recognized in Perrault syndrome, a syndrome that is genetically heterogeneous and associated thus far with mutations in the genes HSD17B4, HARS2, LARS2, CLPP, and C10orf2,3 implicating mitochondrial and peroxisomal pathogenesis in this constellation. Patients with mutations in the mitochondrial tRNA synthase genes LARS2 or KARS in addition to sensorineural hearing loss and ovarian insufficiency may also have cerebral leukodystrophy.29
Possible downstream effects of GGPPS dysfunction and thus GGPP deficiency would be predicted to include impaired geranylgeranylation of small GTPases of the Rab, and Rho/Rac families with consequences on organelles involved in autophagy (such as Rabs23/24/7b), mitochondrial fission and fusion (Rab32), and actin filament dynamics (RhoA/Rac/Cdc42). A defect in the geranylgeranyl prenylation would thus be an intuitive way to tie together some of the multisystemic effects seen in the patients, including in muscle. In support of impaired muscle cell membrane healing as contributing to the dystrophic process with its high CK, we were able to demonstrate significant delays in membrane healing in patient fibroblast MyoD-transformed myoblasts and myotubes. The defective membrane healing could point to deficits of membrane shuttling from the late endosomal compartment to the membrane (such as Rabs11b/11/4) and/or of mitochondria to the site of injury.30, 31 Some of the findings clinically (worsening of the weakness associated with diarrhea), histologically [occasional ragged red and cytochrome oxidase (COX) deficient fibers], and ultrastructurally (enlarged mitochondria) could also point to mitochondrial dysfunction as one of the involved mechanisms, at least for the muscle aspect of the disease in the more severe patients. In ongoing work, however, we have not yet been able to pinpoint a consistent change in the small GTPases or in the overall prenylome of the cells (not shown). Given the localization of the mutations, however, we would speculate that the effects are more subtle and related to the required precise subcellular spatial and temporal resolution of GGPPS depending on the cellular state and needs.
Organism-wide complete inactivation of GGPPS function is not compatible with multicellular survival. A hypomorphic allele at the quemao locus in Drosophila melanogaster causes the bristle hair phenotype.32 A complete knock-out of Ggps1 in the mouse is not viable, so that a floxed allele of Ggps1 in the mouse has been generated,33 allowing for several tissue-specific models to be created.19, 34-38 Although not completely inactivating the enzyme, the resulting GGPPS deficiency state caused a plethora of abnormalities by disrupting the protein FPP/GGPP balance, resulting in both increased protein farnesylation (Rhe-b in cardiac myocyte, H-Ras in Sertoli cell, and Rhe-b in germ cell of testis) and decreased protein geranylgeranylation (K-Ras in lung epithelial cell, RhoA and Rab27 in oocyte, and RhoA in skeletal muscle cell). Interestingly, the MCK-Cre driving muscle-specific GGPPS inactivation did not cause abnormality in muscle fiber morphology, fiber type composition, running ability, or rotarod test but improved systemic insulin sensitivity by activating Irs-1/Pi3k/Akt pathway through decreasing RhoA geranylgeranylation. Since GGPPS enzyme activities of the mutant GGPPS identified in our patients were only mildly reduced, we speculate the function of these GGPPS mutants may be different from general GGPPS activity reduction.
Interference with the mevalonate pathway has been linked to human disease (see Fig 3C). Pharmacological targeting of the mevalonate pathway includes the use of statins to block HMGCoA reductase, which can lead to the development of myopathy in a subset of patients.39 Abnormal geranylgeranylation specifically has been reported in muscle biopsies from patients with statin myopathy as well as in cellular models40 with reduced prenylation of small GTPases including Rab and RhoA, and increased apoptosis.41 The anti–bone-resorptive drug class of bisphosphonates directly inhibit farnesyl diphosphate synthase or GGPPS. Loss of bone-resorptive activity and osteoclast apoptosis is due primarily to loss of geranylgeranylated small GTPases.42 In this context, it is of interest that a rare genetic variant in GGPS1 (Asp188Tyr) has been linked to an increased fracture risk.10, 11, 43 When expressed in cells, the Asp188Tyr variant leads to reduced enzyme activity.12 In our patients, there was no apparent bone phenotype; however, none of the patients has been treated with bisphosphonates, so that it remains unclear whether our patient cohort would react to the drug with higher sensitivity.
The specific biallelic mutations identified in this patient cohort were all extremely rare, recessively acting missense mutations, 3 of which (R261H, R261G, and F257C) were recurrent in different ethnic populations. All mutations except for one allele (P15S in Patient P3) define a stretch of 5 amino acids in the 11th α-helix as a functionally important microdomain that is specific to animal GGPPS and not seen in the plant enzyme. The changes induced by the mutations are not structurally dramatic, but they likely change the hydrogen bonding network within the helix. The domain is clearly outside the catalytic site, which is located within the barrel formed by the 10 helices, but it faces outward, where it could potentially interact with as yet to be defined binding partners involved in the localization or shuttling of the enzyme (see Fig 4A). Only the P15S mutation is outside of the narrow 5 amino acid domain, but it occurs in compound heterozygosity with the R261G mutation in the original domain.44 It is possible that P15S represents a hypomorphic allele acting in conjunction with the R261G missense in the commonly mutated microdomain.
Enzymatic activity of GGPPS in patient-derived fibroblasts was only moderately impaired, to about 50%, a degree of enzymatic dysfunction that in metabolic disease in general should not lead to a phenotype. Heterozygous loss-of-function alleles in GGPS1 are listed in gnomAD in presumably normal individuals. It is thus likely that the effects of the mutations on GGPPS function are more subtle, for instance, by impairing the dynamic subcellular localization of the enzyme for cell-type–specific processes. It is notable that our effort to generate a knock-in mouse of one of the missense mutations was unexpectedly severe in that it caused embryonic lethality, the cause of which is not entirely clear, but strong evidence for a clear underdevelopment of the placental/embryonic vascular unit was evident. Small GTPases, including Rap1, Rho, and Rab GTPases, have previously been reported to regulate cell–cell junctions in various cell types and developmental stages,45, 46 including for vascular and heart morphogenesis.45, 47, 48 Although not providing a suitable model for the human disease, the severity of the phenotype seen in the resulting Y259C knock-in mouse confirms the sensitivity of organisms to GGPPS dysfunction as well as the general pathogenicity of the missense mutation. To address this, we are generating a new mouse line that is heterozygous for the knock-in allele and a floxed allele for tissue-specific “unmasking” of the missense, as well as a knock-in line for a milder missense seen in homozygosity in some of the patients.
Biallelic, highly specific missense mutations in GGPS1 in 11 patients from 6 independent families define a unique new syndrome of muscular dystrophy with associated congenital sensorineural hearing loss and primary ovarian insufficiency in females, while also highlighting the mevalonate/isoprenoid pathway as a novel, albeit still incompletely explored, pathway for muscular dystrophy, hearing loss, and infertility. Because of the fundamental role GGPPS plays in cellular processes at all levels, it is likely that the effects of the mutations described here will be highly specific as to their cellular mechanisms in muscle, inner ear, and ovary. Shedding light on these new mechanisms and pathways might also open avenues for therapeutic intervention.
Acknowledgment
This study was supported by intramural funds from the NIH National Institute of Neurological Disorders and Stroke (grant to C.G.B). J.E.D. is supported by National Institute for Health Research Oxford Biomedical Research Centre, Oxford, United Kingdom. J.L. and J.B. are supported by France Génomique (ANR-10-INBS-09) and Fondation Maladies Rares within the framework of the “Myocapture” sequencing project.
We thank the patients and their families for their generous participation in this study; Dr P. Mohassel for his help in evaluating the patients; and M. Pizzimenti for technical assistance.
Author Contributions
A.R.F., Y.Z., J.R., and C.G.B. contributed to the conception and design of the study; all authors contributed to the acquisition and analysis of data; A.R.F., Y.Z., J.E.D., J.R., N.R., S.Do., M.S., E.M., J.K.J., T.S., and C.G.B contributed to drafting the text and preparing the figures.
Potential Conflicts of Interest
T.V. reports personal fees from DZHK, Antisense Therapeutics, Biophytis, Capricor, Italfarmaco, Santhera, Servier, Sarepta, Solid Biosciences, Dynacure, DiNAQOR, and Catabasis outside the submitted work.




