Neurosurgical Intervention for Supratentorial Intracerebral Hemorrhage
Abstract
Objective
The effect of surgical treatment for supratentorial spontaneous intracerebral hemorrhage (ICH) and whether it is modified by key baseline characteristics and timing remains uncertain.
Methods
We performed a systematic review and meta-analysis of randomized controlled trials of surgical treatment of supratentorial spontaneous ICH aimed at clot removal. We searched MEDLINE, Embase, and Cochrane databases up to February 21, 2019. Primary outcome was good functional outcome at follow-up; secondary outcomes were death and serious adverse events. We analyzed all types of surgery combined and minimally invasive approaches separately. We pooled risk ratios with 95% confidence intervals and assessed the modifying effect of age, Glasgow Coma Scale, hematoma volume, and timing of surgery with meta-regression analysis.
Results
We included 21 studies with 4,145 patients; 4 (19%) were of the highest quality. Risk ratio of good functional outcome after any type of surgery was 1.40 (95% confidence interval [CI] = 1.22–1.60, I2 = 46%, 20 studies), and after minimally invasive surgery it was 1.47 (95% CI = 1.26–1.72, I2 = 47%, 12 studies). For death, the risk ratio for any type of surgery was 0.77 (95% CI = 0.68–0.85, I2 = 23%, 21 studies), and for minimally invasive surgery it was 0.68 (95% CI = 0.56–0.83, I2 = 14%, 13 studies). Serious adverse events were reported infrequently. Surgery seemed more effective when performed sooner after symptom onset (p = 0.04, 12 studies). Age, Glasgow Coma Scale, and hematoma volume did not modify the effect of surgery.
Interpretation
Surgical treatment of supratentorial spontaneous ICH may be beneficial, in particular with minimally invasive procedures and when performed soon after symptom onset. Further well-designed randomized trials are needed to demonstrate whether (minimally invasive) surgery improves functional outcome after ICH and to determine the optimal time window of the treatment after symptom onset. ANN NEUROL 2020;88:239–250.
Acute nontraumatic spontaneous intracerebral hemorrhage (sICH), accounts for 15 to 20% of all strokes in the Western population and for 20 to 50% in developing countries.1-3 ICH is the deadliest stroke subtype, with a 30-day case fatality of approximately 40%.4 Rapid identification and treatment are essential to facilitate recovery.5 However, of patients surviving, only few gain independence.4, 6 Apart from the effect of stroke unit care7 and early control of elevated blood pressure, which may be beneficial,8, 9 there are no treatments with proven benefit.5, 10, 11 A recent study showed that implementation of a hyperacute care bundle (anticoagulation reversal, intensive blood pressure lowering, neurosurgery in selected patients, access to critical care), reduces case fatality.12
The role of surgery in supratentorial sICH remains controversial.13, 14 This is reflected in the American and European guidelines, which refrain from giving firm advice regarding the role of surgery in ICH. As a result, there is large variation in clinical practice.11 The landmark trials STICH and STICH II failed to demonstrate a beneficial effect of surgical treatment, mostly craniotomy, but surgery was performed late, on average 30 hours after symptom onset in STICH15 and 27 hours in STICH II.16 In the STICH trials, crossover from the control arm to surgery was allowed if a patient deteriorated. Increasing evidence suggests that with minimally invasive procedures the potentially adverse effect of open surgery in patients with sICH can be avoided and a beneficial effect on functional outcome may be achieved. An individual patient data meta-analysis of randomized controlled trials (RCTs) published up to 2010 suggested that the effect of surgery may be modified by the clinical state of the patient and the timing of surgery, but in this analysis only a minority of patients were treated with minimally invasive techniques.17 Recently, the MISTIE III trial showed that minimally invasive hematoma aspiration with local application of alteplase up to 72 hours after surgery did not seem to be superior to standard medical care.18 However, surgery in this trial was also performed late, on average 58 hours after symptom onset.18
Approximately one-quarter of patients with ICH show hematoma growth, with the highest probability of growth within the first 3 hours after symptom onset.19 Besides the direct brain injury by compression and disruption of parenchyma, sICH elicits a secondary response. This secondary brain injury results from toxicity due to blood degradation products (eg, heme, iron) and plasma-derived components (eg thrombin), which starts within 3 to 4 hours after sICH, causing an inflammatory response and the development of perihematomal edema.20 Hematoma volume, hematoma growth, and perihematomal edema are independent predictors of poor outcome.21, 22 Targeting hematoma growth, inflammation, and perihematomal edema at an early stage after sICH may reduce not only hematoma volume but also secondary brain injury, and could possibly improve outcome. However, a previous pilot study of “ultra-early” surgery within 4 hours after sICH in 20 patients was stopped early after a planned interim analysis in 11 patients because of postoperative bleeding in 4 patients, in 3 of them fatal.23 Others found no difference in rebleeding rates between stereotactic treatment within (mean = 4.8 h, 32 patients, 1 rebleed), or after 6 hours (mean = 13.8 h, 27 patients, 2 rebleeds) from symptom onset in computed tomography (CT) angiographic spot sign–negative patients, suggesting that early surgery may be safe in patients with ICH in the absence of a spot sign.24
We aimed to systematically review and meta-analyze RCTs of surgical treatment of supratentorial sICH aimed at clot removal, both overall and for minimally invasive treatment separately, and to assess the modifying effect of age, Glasgow Coma Score (GCS), hematoma volume, and timing of surgery.
Patients and Methods
We adhered to the PRISMA guidelines25 and registered the study protocol in PROSPERO (CRD42018098864).
Search Strategy and Selection Criteria
We searched MEDLINE, Embase, and Cochrane databases up to February 21, 2019. We included RCTs on the effect of neurosurgical hematoma evacuation, compared with standard medical management on functional outcome and death, in adult patients (≥18 years of age) with a CT or magnetic resonance scan-confirmed supratentorial sICH. Neurosurgical intervention could consist of craniotomy, craniopuncture, stereotactic aspiration, endoscopy-guided aspiration with or without local clot mobilization techniques using thrombolytic agents, or alternative methods. Treatment of hydrocephalus with extraventricular drainage (EVD) alone was not included as an intervention of interest and was allowed in both groups. Duration of follow-up in the trial had to be at least 3 months. We excluded studies that included patients with secondary causes of ICH (ie, hemorrhage due to trauma, aneurysm, arteriovenous malformation and dural arteriovenous fistula, cavernous malformation and tumor) or infratentorial hemorrhages (unless separately reported) and if ≥15% of the surgical interventions were decompressive hemicraniectomies without hematoma removal. Conference abstracts were excluded. We did not apply any language restriction. The search strategy consisted of terms for (supratentorial) ICH and terms for surgical treatment or hematoma evacuation. We identified further studies from the reference lists of included papers and screened reference lists of review articles. Finally, we searched ClinicalTrials.gov, the International Standard Randomized Controlled Trial Number registry, and the https://www.isrctn.com/ database for clinical trials, and ClinicalTrialsRegister.eu for unpublished studies.
Two authors (L.S. and F.H.B.M.S.) independently screened the abstracts and assessed full texts to identify studies that potentially met the predefined inclusion and exclusion criteria. Any disagreements between these authors were resolved by a third reviewer (C.J.M.K.). For articles written in Chinese, we received help from 2 native Chinese colleagues.
Data Synthesis and Statistical Analyses
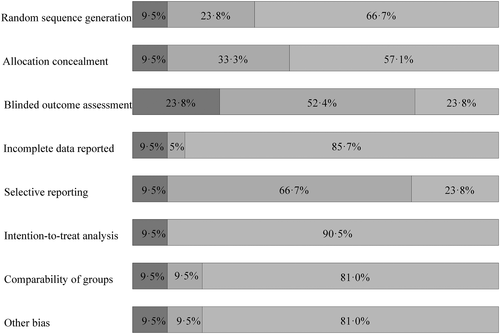
Two authors (L.S. and F.H.B.M.S.) independently extracted data from the included studies, using a standardized, prepiloted form, and assessed the risk of bias using the following items from the Cochrane criteria: random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective reporting, and performance of an intention-to-treat analysis.26 Because blinding to treatment of participants and personnel is not feasible in a neurosurgical trial, we discarded this item. Disagreements on extracted data were resolved by discussion with a third reviewer (C.J.M.K.).
We extracted the following baseline characteristics: sex, mean or median age and age range, clinical condition on admission measured by the mean or median GCS and range, proportion with a history of hypertension, ICH location, mean or median ICH volume and range, presence of intraventricular hemorrhage, surgical technique, mean or median time from symptom onset to surgery and range, and proportion of patients who crossed over from control to intervention.
The prespecified primary outcome was good functional outcome at 3-month follow-up. Because the timing of the primary outcome event in the included studies varied from 3 to 12 months, we assessed whether the effect size of surgery differed in studies reporting outcomes at 3, 6, and 12 months. Because the estimates of surgery appeared similar for all time points, we combined all studies, using 6 months as the preferred time point (available in most studies), and we used 3 or 12 months if the 6-month outcome was not provided. We defined good functional outcome as a modified Rankin Scale score of 0 to 3, a Glasgow Outcome Scale score of 4 to 5, an extended Glasgow Outcome Scale score of 5 to 8, or a Barthel Index (BI) of ≥60. In studies that did not report any of these outcomes, we used the scale and cutoff points for good functional outcome as reported by the authors.
Secondary outcomes were death at 6 months (or when not available at 3 or 12 months), case fatality at 30 days, and serious adverse events within 30 days, including rebleeding, epileptic seizures, intracranial infection, hydrocephalus, severe systemic infection, gastrointestinal bleed, need for (repeated) surgical intervention, and EVD.
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for good functional outcome and death at the time of follow-up, using a random effects model for all surgical procedures, for studies on minimally invasive surgery, and for studies on (>99%) craniotomy. We performed a sensitivity analysis assessing high-quality studies only, defined as studies with a low risk of bias on all items as determined by the Cochrane risk of bias tool. We assessed heterogeneity by means of the I-squared statistic (I2) and categorized heterogeneity as follows: 0 to 40%, heterogeneity that might not be important; 30 to 60%, moderate heterogeneity; 50 to 90%, substantial heterogeneity; and 75 to 100%, considerable heterogeneity. In addition, we calculated prediction intervals, which help in the clinical interpretation of heterogeneity by estimating the expected range of true effects in similar studies. They can be used as a tool for interpreting evidence and enable more informed clinical decision-making.27 We performed a sensitivity analysis for good functional outcome at follow-up, excluding the 2 studies that used BI as outcome measure. We constructed funnel plots to evaluate potential publication bias.
We performed meta-regression analysis for the primary outcome to assess the effect of 4 prespecified factors that have previously been suggested to influence the effect of surgery15-17: age (mean, or when not available median age), GCS (median, or when not available mean GCS), time from symptom onset to surgery, and ICH volume (mean, or when not available median ICH volume). We performed meta-regression for these potential modifying factors only if there were at least 10 studies available that reported that specific factor; further multivariate meta-regression analysis was performed if at least 10 studies were available for every modifying factor included (eg, 20 studies for analysis of 2 factors).26, 28, 29 In addition, we assessed the effect of surgery in subgroups according to ICH location (lobar vs nonlobar), because the effect of surgery appeared to be different in lobar than in deep ICH in STICH.15 Finally, we summarized the reported percentages of adverse events.
We used the software program R and R-studio version 3.4.4, packages “meta,” “metafor,” and “foreign.”
Results
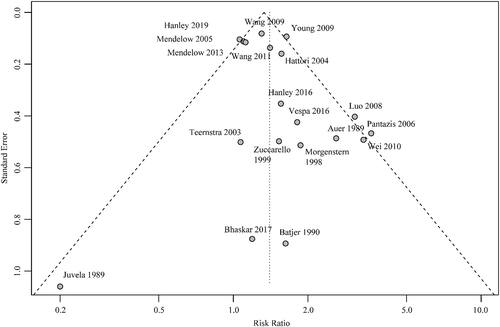
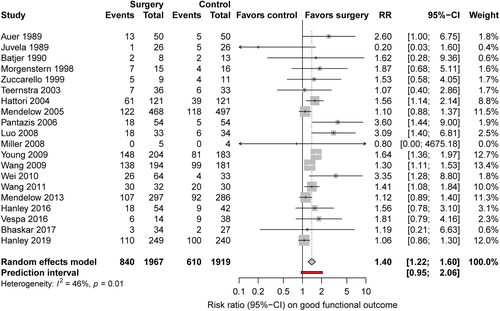
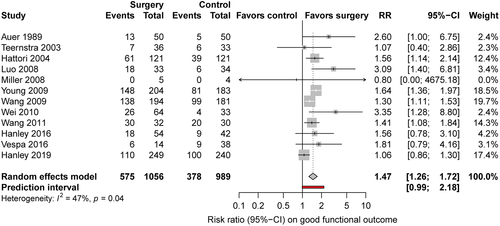
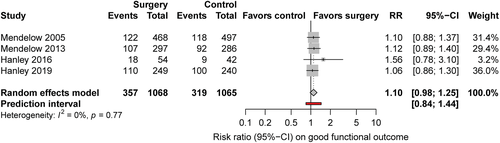
We identified 21 studies (Fig 1) including 4,145 patients, of whom 2,091 were randomized for neurosurgical hematoma evacuation and 2,054 patients for standard medical management. One eligible study did not provide data on functional outcome and was excluded for the meta-analysis on good functional outcome.30 The Table summarizes the characteristics of the included studies. Inclusion criteria for the individual RCTs are listed in the supplement (Supplementary Table 4). Of the 21 studies, 13 studies assessed minimally invasive surgical approaches,18, 30-32, 36-42, 44, 45 in 6 of them in combination with a thrombolytic agent.18, 31, 37, 41, 42, 45 One study included patients with lobar ICH only,16 and 7 studies were restricted to patients with deep ICH.30, 34, 38, 39, 41, 42, 44 Nine studies specified whether thalamic hemorrhages were included.16, 18, 31, 33-36, 39, 43 Four studies included 12 to 23% thalamic hemorrhages,33, 35, 36, 39 and 3 did not include thalamic hemorrhages.16, 34, 43 Intraventricular extension of ICH was an exclusion criterion in 1 study.16 Quality of studies was low in 9 studies,30, 32, 34-36, 38-40, 45and moderate in 8 studies.33, 37, 41-44, 46, 47 Only 4 studies were of high quality,15, 16, 18, 31 of which 2 investigated minimally invasive surgery (Fig 2).18, 31 The funnel plot suggested possible publication bias for smaller studies demonstrating results in favor of medical management (Fig 3). Six to 26% of patients randomized for standard medical management crossed over to surgical hematoma evacuation (10 studies).15, 16, 18, 31, 33, 38, 39, 41, 43, 46 Data on functional outcome were available in 1,967 (99.5%) patients randomized to surgery and in 1,919 (99.3%) patients randomized to standard medical management. After surgical treatment, the chance of good functional outcome was 40% higher than after medical management (RR = 1.40, 95% CI = 1.22–1.60, I2 = 46%; Fig 4). Estimates for subgroups of studies with outcome assessment at 3, 6, and 12 months are listed in Appendix S1 (see Supplementary Table 1). Surgical treatment also lowered the risk of death (RR = 0.77, 95% CI = 0.68–0.85, I2 = 21%, 21 studies) at the time of follow-up. The RR for 30-day case fatality after any type of surgery was 0.68 (95% CI = 0.51–0.92, I2 = 0%, 5 studies). The chance of good functional outcome after minimally invasive surgery was 47% higher than after medical management (RR = 1.47, 95% CI =1.26–1.72) with moderate heterogeneity (Fig 5). Estimates for subgroups of studies with outcome assessment at 3, 6, and 12 months were similar. Minimally invasive surgery lowered the risk of death (RR = 0.68, 95% CI = 0.56–0.83, I2 = 14%, 13 studies) at the time of follow-up. The RR for 30-day case fatality after minimally invasive surgery was 0.65 (95% CI = 0.41–1.02, I2 = 0%, 3 studies). In studies investigating craniotomy, RR for good functional outcome after surgery was 1.44 (95% CI = 0.69–2.93, I2 = 48%, 6 studies), RR for death was 0.79 (95% CI = 0.66–0.94, I2 = 0%, 6 studies), and RR for 30-day case fatality was 0.73 (95% CI = 0.46–1.15, I2 = 26%, 2 studies; Supplementary Table 2). When we restricted the analyses to high-quality studies (2 studies assessing mostly craniotomy,15, 16 2 assessing stereotactic surgery,18, 31 including a total of 2,166 patients), RRs for good functional outcome (RR = 1.10, 95% CI = 0.98–1.25, I2 = 0%; Fig 6) and death (RR = 0.86, 95% CI = 0.72–1.03, I2 = 3%, 4 studies) no longer showed a statistically significant effect of surgical treatment. Sensitivity analyses excluding 2 studies that used BI as outcome measure42, 46 showed similar results for the chance of good functional outcome at follow-up (any type of surgery: RR = 1.43, 95% CI = 1.22–1.69, I2 = 51%, 18 studies; minimally invasive surgery: RR = 1.54, 95% CI = 1.27–1.87, I2 = 49%, 11 studies).
| Study | P, n | C, n | Type of Surgery | Use of Thrombolytic Agent, % of Patients | Age, Mean yr | Male, n (%) | GCS, Median (IQR) | Deep ICH, n (%) | Onset to Surgery, Mean h | IVH, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Auer 198936 | 50 | 50 | Endoscopy-guided | No | NR | 61 (61) | NR | 55 (55) | NR | NR |
| Juvela 198935 | 26 | 26 | Craniotomy | NA | 52 | 30 (58) | 12 (7–14) | 44 (85) | 14.5a | 32 (62%) |
| Batjer199034 | 8 | 13 | Craniotomy | NA | 55 | NR | NR | 21 (100) | NR | NR |
| Morgenstern 199846 | 17 | 17 | Craniotomy | NA | 51b | 22 (65) | 11 (10–14) | 26 (76) | 8.3a | NR |
| Zuccarello 199933 | 9 | 11 | Craniotomy, n = 5; stereotactic, n = 4 | Urokinase in stereotactic | 62 | 11 (55) | 12 (9–14) | 10 (50) | 8.6a | 11 (55%) |
| Teernstra 200337 | 36 | 34 | Stereotactic | Urokinase (100%) | 68 | 40 (57) | 9 (7–11) | 32 (46) | 12 | 23 (33%) |
| Hattori 200444 | 121 | 121 | Stereotactic | No | 61 | 148 (61) | NR | 242 (100) | NR | NR |
| Mendelow 200515 | 503 | 530 | Craniotomy 75% | NR | 62b | 591 (57) | 12 (9–14) | 434 (42) | 30a | NR |
| Pantazis 200643 | 54 | 54 | Craniotomy | NA | 61 | 60 (56) | 9c | 57 (53) | 6.2a | NR |
| Cho 200830 | 113 | 113 | Endoscopy-guided | No | 63 | 151 (67) | 11 | 226 (100) | NR | 72 (32%) |
| Luo 200839 | 36 | 39 | Aspiration | No | 55 | 44 (59) | NR | 75 (100) | NR | NR |
| Miller 200840 | 6 | 4 | Endoscopy-guided | No | 59 | 9 (90) | NR | 1 (10) | 18 | 6 (60%) |
| Young 200941 | 204 | 183 | Stereotactic | Urokinase in 26% | 66 | 289 (75) | 14 (14–15) | 387 (100) | NR | NR |
| Wang 200942 | 195 | 182 | Stereotactic | Urokinase (100%) | 56 | 236 (63) | 12 (10–14) | 377 (100) | 21 | 70 (19%) |
| Wei 201045 | 67 | 39 | Various techniquesd | Urokinase (100%) | 57b | 58 (55) | NR | NR (60) | NR | NR |
| Wang 201138 | 32 | 30 | Craniopuncture | No | 46 | 34 (55) | NR | 62 (100) | 8.2 | NR |
| Mendelow 201316 | 305 | 292 | Craniotomy 99% | NA | 64 | 340 (57) | 13 (12–15) | 0 (0) | 26.7 | 0 (0%) |
| Hanley 201631 | 54 | 42 | Stereotactic | Alteplase (85%) | 61 | 63 (66) | NR | 63 (66) | NR | NR |
| Vespa 201632 | 14 | 42 | Endoscopy-guided | No | NR | 37 (66) | NR | 39 (70) | 29.9a | NR |
| Bhaskar 201747 | 34 | 27 | Craniotomy | NA | 55 | 37 (61) | NR | 52 (85) | NR | 31 (51%) |
| Hanley 201918 | 250 | 249 | Stereotactic | Alteplase (94%) | NR | 305 (61) | 62 (52–70) | 307 (62) | 58.3 | NR |
- a Median time between symptom onset and surgery.
- b Data reported as median age.
- c Data reported as mean GCS.
- d Small skull window microsurgery was performed in 31 patients and minimally invasive surgery fragmenting and aspirating hematoma in 36 patients.
- C = controls; GCS = Glasgow Coma Scale; ICH = intracerebral hemorrhage; IQR = interquartile range; IVH = intraventricular hemorrhage; NA = not applicable; NR = not reported; P = patients, surgically treated.
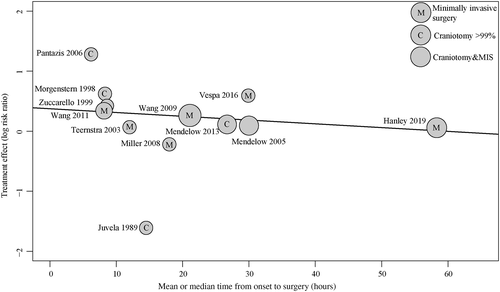
We found no statistically significant modifying influence of age (18 studies, p = 0.61), GCS score on admission (13 studies, p = 0.97), or ICH volume (15 studies, p = 0.50) on good functional outcome at the time of follow-up (Supplementary Table 3). Surgery seemed more effective than medical management alone when performed sooner after the onset of symptoms (12 studies, p = 0.04; Fig 7). We found no modifying effects of age (19 studies, p = 0.43), GCS (14 studies, p = 0.29), ICH volume (15 studies, p = 0.32), or timing of surgery (12 studies, p = 0.37) on death. Because any combination of modifying factors was reported in <20 studies, we refrained from further multivariate meta-regression analysis.26, 28, 29 The number of studies that assessed minimally invasive surgery and reported modifying factors was <10, which precluded meta-regression analysis in this subgroup. In the subgroup of patients with deep ICH, the RR of good functional outcome after surgery compared to medical management was 1.51 (95% CI = 1.31–1.75, I2 = 25%, 9 studies), and in those with lobar hemorrhages it was 1.25 (95% CI = 0.92–1.71, I2 = 13%, 4 studies). Reporting of adverse and serious adverse events varied among studies (Supplementary Table 5). In surgically treated patients, a second surgical procedure was needed in 4 to 16% of patients (5 studies).15, 18, 31, 33, 41 Rebleeding occurred in 0 to 50% of medically treated patients (12 studies),16, 18, 31-33, 36, 38-40, 42, 43, 45 and in 0 to 35% of surgically treated patients (13 studies).16, 18, 31-33, 36-40, 42, 43, 45 Three studies reported a higher frequency of rebleeds in the group randomized to medical management than in those who received surgery,16, 36, 45 whereas 5 studies found more rebleeds in the group that underwent hematoma evacuation.18, 31, 32, 37, 42 Four of these 5 studies used local application of a thrombolytic agent to optimize the evacuation of the hematoma.18, 31, 37, 42 Operative site infections were rare.18, 31-33, 38
Discussion
Our meta-analysis shows that neurosurgical hematoma evacuation in patients with supratentorial sICH holds promise to improve functional outcome and death at 3 to 12 months, but only the minority of studies was without bias. The effect of hematoma evacuation appears most prominent with application of minimally invasive techniques and when performed sooner rather than later after symptom onset. We found no modifying effect of age, clinical state on admission, and ICH volume. In the 4 RCTs of high quality, the effect of neurosurgical hematoma evacuation on functional outcome and on death was no longer statistically significant.
One of the important factors for why earlier high-quality RCTs may have failed to demonstrate a beneficial effect of surgical hematoma evacuation for supratentorial sICH is the timing of the surgical treatment. In the previous individual patient data meta-analysis of RCTs published up to 2010 that suggested that earlier surgery may increase the benefit of intervention, the time interval between symptom onset and randomization in the included studies was still long.17 Approximately 40% of patients who had a known time interval from onset to randomization were randomized within 8 hours, approximately 30% between 8 and 24 hours, and approximately 30% >24 hours after symptom onset. Time intervals between symptom onset and surgery were not reported, but most likely only few patients will have had their hematoma evacuation within 6 hours after symptom onset in all trials, and even fewer within 3 hours after symptom onset.48 Compared to the individual patient data meta-analysis, we were able to include more studies,16, 18, 30-32, 34, 38-41, 43-45, 47 among which are studies with earlier treatment38, 40 and studies that tested minimally invasive approaches.18, 30-32, 38-41, 44, 45 As one-quarter of patients with ICH show hematoma growth consistent with ongoing bleeding in the first hours after symptom onset, some neurosurgeons advocate postponing surgical intervention until the hematoma has stabilized.23, 24 The nonrandomized pilot study of “ultra-early” open craniotomy and hematoma evacuation (median time to surgery = 180 minutes) showed rebleeding in 4 of 11 patients and was therefore stopped early.23 A recent study suggested that stereotactic aspiration within 6 hours after symptom onset may be safe in patients with sICH in the absence of a spot sign.24 However, it should be noted that in a recent individual patient data meta-analysis of 5,435 patients assessing prediction of hematoma growth, the addition of the CT angiography spot sign to a prediction model with time from symptom onset, ICH volume, anticoagulant use, and antiplatelet use improved the C-index only slightly (from 0.78, 95% CI = 0.75–0.82 to 0.83, 95% CI = 0.80–0.86).19 The risks of early hematoma growth and rebleeding after ultra-early surgery have shaped the inclusion criterion of proof of a stable ICH volume before starting any type of surgery in recent and ongoing RCTs (MIND, NCT03342664)18, 31, 32 and an ongoing nonrandomized trial (INVEST, NCT02654015). However, theoretically early surgery could also have additional benefits, such as prevention of ICH growth, additional mass effect and raised intracranial pressure, and amelioration of the secondary brain injury resulting from the toxic effect of blood degradation products and the inflammatory response after ICH. If the timing of surgery is a key success factor, this would favor minimally invasive techniques without the use of thrombolytic agents over procedures that include a time-consuming thrombolysis of the hematoma to reach optimal evacuation. In MISTIE III, surgical treatment was started on average 58.3 hours after symptom onset, the first dose of alteplase was administered after on average 72.6 hours, and treatment was completed after on average after 123 hours.18 The prespecified subgroup analysis on timing in the MISTIE III trial showed no effect of timing of treatment, but none of the patients was treated before 18 hours after symptom onset.18 A recent systematic review comparing randomized controlled trials of minimally invasive surgery versus conventional craniotomy, or versus medical management, showed similar benefits for minimally invasive surgery performed within 24 hours as for surgery performed within 72 hours.49 However, this analysis differs from ours in that it also included studies of minimally invasive surgery compared to conventional craniotomy and by their method of analyzing timing of surgery, including the patients treated within 24 hours also in the group treated within 72 hours. The results from our meta-regression support a potential beneficial effect from earlier surgery, but it is important to note that the average time to surgery in all studies but one43 was >8 hours. Furthermore, results of any meta-regression analysis should be interpreted with caution, because they are observational associations based on averages at the study level and not based on individual patient data, and the included studies have different inclusion and exclusion criteria. Based on the combined evidence of this systematic review and meta-regression and that of others, further well-designed randomized studies should be performed in both the earlier and later time windows. The Dutch ICH Surgery Trial pilot study (NCT03608423; part of the CONTRAST consortium; Dutch-ICH.nl) is currently assessing safety, technical effectiveness, and feasibility of minimally invasive endoscopy-guided surgery for sICH within 8 hours of symptom onset, whereas others are assessing the effect of minimally invasive surgery within 24 hours (MIND, NCT03342664; INVEST, NCT02654015; ENRICH, NCT02880878). Early treatment after sICH may pose a logistical challenge in particular in countries with large distances between patients and neurosurgical centers. Public policies of reimbursement of treatment only for patients who are included in a randomized controlled trial may boost recruitment rates.50
In contrast with the previous individual patient data meta-analysis,17 we did not find a modifying effect of age or the clinical state of the patient at admission. Based on the results of our meta-regression analysis, we suggest that there is no reason to exclude elderly patients from inclusion in future RCTs, or to restrict inclusion to patients on the basis of their GCS score. However, meta-regression analysis is based on observational relations using averages of potential modifying factors within studies and accordingly limited ranges of the modifying factors. Therefore, the observed relations across studies can be subject to confounding by other characteristics that vary between the studies, and relations may not be undoubtedly causal (ecological bias). Furthermore, the small number of studies that reported modifying factors precluded multivariate meta-regression analysis. Individual patient data meta-analysis of all studies included in our meta-analysis could strengthen our findings. STICH suggested a potential modifying effect of sICH location in favor of lobar hemorrhages over deep sICH,15 but this could not be confirmed by STICH II,16 nor by the individual patient data meta-analysis.17 In contrast, our subgroup analysis showed a beneficial effect of hematoma evacuation in patients with deep hemorrhages, but not in those with lobar hemorrhages. A possible explanation is that the modifying effect of location may vary between craniotomy and minimally invasive hematoma evacuation.
Our study has several strengths. First, we performed a comprehensive literature search without any language or publication date restrictions. Second, we assessed the effect of surgery aimed at hemorrhage evacuation overall, as well as of minimally invasive techniques separately. Third, we performed a sensitivity analysis in high-quality studies and were able to assess modifying factors through meta-regression analysis. Finally, in contrast to previous systematic reviews on minimally invasive surgery,49, 51, 52 we excluded studies that compared minimally invasive techniques to craniotomy.
Our study also has limitations, the most important being the moderate to low quality of some of the included studies. Only 5 studies performed blinded outcome assessments,15, 16, 18, 31, 44 and only 4 studies were of high quality overall.15, 16, 18, 31 In addition, crossover to surgery was high in some studies,15, 16, 38, 39 and not reported in many.32, 34-37, 40, 42, 44, 45, 47 Another limitation is that studies varied with respect to timing of both surgery and outcome assessment, the definition of good functional outcome, and the reporting of adverse events. Finally, the use of averages of potential modifying factors in studies, and not individual patient data in meta-regression analysis, could have led to ecological bias. Because of this, we refrained from translating the association to an estimate in terms of a quantitative effect per hour earlier that surgery is commenced. Individual patient data meta-analysis of the published RCTs could further inform us on whether a larger benefit may be expected in specific subgroups, with specific surgical approaches, or in a specific time-window, without having the possible disadvantages of a meta-regression analysis.
Taken together and despite the recent neutral results of MISTIE III, the results of our systematic review and meta-analysis suggest that surgical treatment may be beneficial to improve outcome in patients with supratentorial sICH, in particular with minimally invasive procedures and when performed early after symptom onset. However, because of the methodological shortcomings and high risk of bias of most included studies, hematoma evacuation cannot be recommended as standard treatment until further high-quality RCTs have firmly proven its effect. Such trials should have a large sample size, high adherence to the intervention, focused hypothesis-testing, and blinded outcome assessment.53 Physicians involved in the care of patients with ICH should be encouraged to enroll patients with a supratentorial sICH in trials investigating the benefit of (minimally invasive) surgery.
Acknowledgment
We acknowledge the support of the Netherlands Cardiovascular Research Initiative, which is supported by the Dutch Heart Foundation (CVON2015-01: CONTRAST) and the Brain Foundation Netherlands (HA2015.01.06). C.J.M.K. and F.H.B.M.S. are supported by a clinical established investigator grant of the Dutch Heart Foundation (2012 T077) and C.J.M.K. by an ASPASIA grant from the Netherlands Organization for Health Research and Development (ZonMw; 015008048).
We thank Drs W. Sun and B. Fan for their help translating the Chinese articles.
The Dutch ICH Surgery Trial Study Group approved this article.
Author Contributions
L.S., F.H.B.M.S., H.D.B., M.M.R., R.D., and C.J.M.K. contributed to the conception and design of the study; L.S., F.H.B.M.S., M.M.R., R.D., and C.J.M.K. contributed to the acquisition and analysis of data; all authors contributed to drafting the text and preparing the figures.
The Dutch ICH Surgery Trial Study Group consists of J. Coutinho, MD, PhD, Amsterdam UMC, University of Amsterdam, Amsterdam, the Netherlands; Prof D. Dippel, MD, PhD, Erasmus MC University Medical Center, Rotterdam, the Netherlands; W. A. Moojen, MD, PhD, Haaglanden Medical Center, the Hague, the Netherlands; J. Boiten, MD, PhD, Haaglanden Medical Center, the Hague, the Netherlands; I. van den Wijngaard, MD, PhD, Haaglanden Medical Center, the Hague, the Netherlands; R. W. Koot, MD, PhD, Leiden University Medical Center, Leiden, the Netherlands; Prof M. J. H. Wermer, MD, PhD, Leiden University Medical Center, Leiden, the Netherlands; O. Teernstra, MD, PhD, Maastricht University Medical Center, Maastricht, the Netherlands; I. de Ridder, MD, PhD, Maastricht University Medical Center, Maastricht, the Netherlands; K. H. Kho, MD, Medical Spectrum Twente, Enschede, the Netherlands; P. J. A. M. Brouwers, MD, PhD, Medical Spectrum Twente, Enschede, the Netherlands; B. van der Pol, MD, PhD, Elisabeth–Two Cities Hospital, Tilburg, the Netherlands; P. L. M. de Kort, MD, PhD, Elisabeth–Two Cities Hospital, Tilburg, the Netherlands; Prof A. van der Zwan, MD, PhD, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, the Netherlands; Prof L. J. Kappelle, MD, PhD, Brain Center Rudolf Magnus, University Medical Center Utrecht, Utrecht, the Netherlands; H. M. den Hertog, MD, PhD, Isala Hospital, Zwolle, the Netherlands; D. Nanda, MD, PhD, Isala Hospital, Zwolle, the Netherlands (http://www.dutch-ich.nl).




