Clinical Outcomes Depending on Acute Blood Pressure After Cerebral Hemorrhage
Abstract
Objective
To determine the association between clinical outcomes and acute systolic blood pressure (SBP) levels achieved after intracerebral hemorrhage (ICH).
Methods
Eligible patients who were randomized to the ATACH-2 (Antihypertensive Treatment in Intracerebral Hemorrhage 2) trial (ClinicalTrials.gov: NCT01176565) were divided into 5 groups by 10-mmHg strata of average hourly minimum SBP (<120, 120–130, 130–140, 140–150, and ≥ 150 mmHg) during 2 to 24 hours after randomization. Outcomes included: 90-day modified Rankin Scale (mRS) 4 to 6; hematoma expansion, defined as an increase ≥6 ml from baseline to 24-hour computed tomography; and cardiorenal adverse events within 7 days.
Results
Of the 1,000 subjects in ATACH-2, 995 with available SBP data were included in the analyses. The proportion of mRS 4 to 6 was 37.5, 36.0, 42.8, 38.6, and 38.0%, respectively. For the “140 to 150” group relative to the “120 to 130,” the odds ratio (OR), adjusting for sex, race, age, onset-to-randomization time, baseline National Institutes of Health Stroke Scale score, hematoma volume, and hematoma location, was 1.62 (95% confidence interval [CI], 1.02–2.58). Hematoma expansion was identified in 16.9, 13.7, 21.4, 18.5, and 26.4%, respectively. The 140 to 150 (OR, 1.80; 95% CI, 1.05–3.09) and “≥150” (1.98; 1.12–3.51) showed a higher frequency of expansion than the 120 to 130 group. Cardiorenal events occurred in 13.6, 16.6, 11.5, 8.1, and 8.2%, respectively. The 140 to 150 (0.43; 0.19–0.88) and ≥ 150 (0.44; 0.18–0.96) showed a lower frequency of the events than the 120 to 130.
Interpretation
Beneficial effects of lowering and maintaining SBP at 120 to 130 mmHg during the first 24 hours on clinical outcomes by suppressing hematoma expansion was somewhat offset by cardiorenal complications. ANN NEUROL 2019;85:105–113.
Although the age-standardized mortality rate for hemorrhagic stroke, including intracerebral hemorrhage (ICH) and subarachnoid hemorrhage, has decreased worldwide over the past 2 decades, incidence, number of deaths, and number of disability-adjusted life-years lost continue to increase.1 Despite showing half the incidence of ischemic stroke globally, hemorrhagic stroke causes more deaths and disability-adjusted life-years lost than ischemic stroke. Furthermore, the impact of acute therapy for ICH lags far behind that for acute ischemic stroke.2, 3
Elevated blood pressure (BP) is common after acute ICH and is associated with poor outcomes.4, 5 The INTERACT2 (Second Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial) demonstrated better functional outcomes with no harm for subjects with acute ICH with early intensive lowering to targeted systolic BP (SBP) of <140 mmHg than the standard lowering to SBP <180 mmHg, although the primary efficacy outcome of death or disability (defined as modified Rankin Scale [mRS] score 3–6) was not statistically significantly different between treatment groups.6 The ATACH (Antihypertensive Treatment of Acute Cerebral Hemorrhage)-2 trial tested similar SBP-targeted groups,7 yet the primary efficacy outcome of mRS 4 to 6 was identical between groups.8 This discrepancy has created a conundrum in recommending optimal SBP goals after acute ICH.9, 10
The discrepancy might be partly attributable to different levels of actual SBP reduction between the 2 trials. In the INTERACT2, hourly mean SBP achieved within the initial day was roughly 140 mmHg in the intensive treatment group and roughly 150 mmHg in the standard treatment group.6 In the ATACH-2, hourly minimum SBPs achieved were roughly 120 and 140 mmHg, respectively,8 and hourly mean SBPs were ≈8 mmHg higher than hourly minimum SBP levels, as described later. The optimal SBP goal would thus lies between 120 and 140 mmHg based on the results from these 2 major randomized clinical trials.
The aim of the present exploratory as-treated analysis of the ATACH-2 is to determine the association between clinical and radiological outcomes, as well as cardiorenal adverse events (AEs), and acute SBP achieved after ICH, in an attempt to clarify the optimal SBP goal for subjects with acute ICH.
Materials and Methods
The ATACH-2 was an international, randomized, 2-group, open-label trial to determine the efficacy of rapidly lowering SBP in patients with hyperacute spontaneous supratentorial ICH (ClinicalTrials.gov: NCT01176565; the University Hospital Medical Information Network clinical trial registry in Japan: UMIN 000006526).8 A detailed description of the protocol has been provided elsewhere.7 Briefly, patients with Glasgow Coma Scale score ≥ 5 and SBP ≥180 mmHg on arrival at the emergency department and intraparenchymal hematoma showing a volume < 60 cm3 on initial non-contrast-enhanced computed tomography (CT) were centrally randomized to undergo either intensive SBP lowering (target SBP, 110–139 mmHg) or standard SBP lowering (target SBP, 140–179 mmHg) in a 1:1 ratio within 4.5 hours of symptom onset. Intravenous nicardipine infusion was initiated to reduce hourly minimum SBP to the target level within 2 hours of randomization and to maintain this level through 24 hours for each subject. Informed consent was obtained from each subject, his or her legally authorized representative, or next of kin.
SBP was measured at least every 30 minutes during the period from 2 to 24 hours after randomization, and minimum and maximum SBPs were recorded each hour. Subjects were divided into 5 groups in 10-mmHg strata of their average hourly minimum SBP, regardless of the randomized treatment. Subjects were also divided into 5 groups based on the average hourly mean SBP between 2 and 24 hours. Concrete SBP levels for grouping were determined to divide subjects as evenly as possible. As additional analyses, subjects were divided into 5 groups in 18-mmHg strata of the absolute reduction of average hourly minimum SBP from the initial SBP at emergent visit, and also divided into 5 groups in 6% strata of their relative reduction of average hourly minimum SBP from the initial SBP at emergent visit.
Neurological status was assessed using the National Institutes of Health Stroke Scale (NIHSS) score at baseline by a qualified study investigator. The trial mandated a head CT at 24 (±8) hours after initiation of treatment. CT images were forwarded to the core imaging analysis center for post-hoc computerized volumetric analysis by neuroimaging specialists who were blinded to the treatment assignment, clinical findings, and other CT results from different time points. Postdischarge follow-up included telephone contact at 1 month to collect data on serious AEs and death, and an in-person clinical evaluation at 90 days to perform physical and neurological examinations and to assess functional disability using the mRS by a qualified investigator who did not participate in the randomization, treatment, or in-hospital clinical management.
Outcome measures for these exploratory analyses are: (1) the proportion of subjects with mRS 4 to 6 at 90 days; (2) death within 90 days; (3) hematoma expansion (HE), defined as an increase in volume > 6 cm3 on the 24-hour CT compared with the baseline CT; and (4) renal and cardiac AEs within 7 days. Of the 5 grades of severity (mild, moderate, severe, life-threatening, and fatal), mild AEs were excluded from the analysis. Linear trends of outcomes among the 5 SBP groups were estimated using the Cochran–Armitage trend test. Comparisons of outcomes among the 5 SBP groups, using logistic regression model with the group containing the largest number of subjects set as a reference, were adjusted for sex, race (Asian), baseline age, onset-to-randomization time, baseline NIHSS score, baseline hematoma volume, and hematoma location (lobar); quartile categories were used for continuous factors. Treatment assignment (the intensive treatment group or the standard treatment group) was also included as a covariable in an additional analysis. Associations between primary and secondary outcomes were estimated using the Wald's chi-square test. The PROC GENMOD procedure of the latest version of SAS software and JMP (version 12.0.1) software (SAS Institute Inc., Cary, NC) were used to obtain test statistics and results. Values of p < 0.05 were considered statistically significant. Because all analyses are considered exploratory and hence no definitive inferences are made regarding the relationship between the SBP levels and outcomes, no adjustments of type I error probability were considered. Furthermore, no imputations were made for missing data.
Results
A total of 1,000 patients were randomized in the ATACH-2. Of these, 5 were excluded from the present analyses because of a lack of available data on their SBPs between 2 and 24 hours. Of the remaining 995, 379 were women, 558 were Asians, and subjects had a mean age 62.0 ± 13.0 years.
Analysis Using Average Hourly Minimum SBP Levels
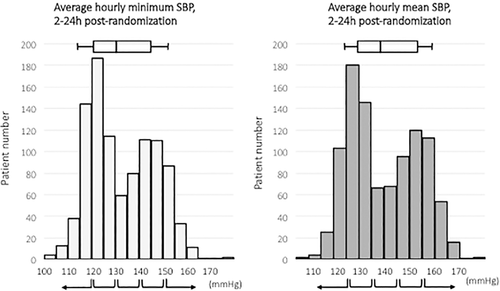
Distribution of average hourly minimum SBP between 2 and 24 hours is shown in Figure 1 (left). Median average hourly minimum SBP was 129.8 mmHg (interquartile range [IQR], 121.1–144.8). Subjects were divided into the following 5 groups based on SBP: <120 (n = 199); 120 to 130 (n = 301); 130 to 140 (n = 139); 140 to 150 (n = 221); and ≥ 150 mmHg (n = 135).
Baseline characteristics of subjects in the 5 groups are shown in Table 1. Age tended to differ (p = 0.053), and hematoma volume differed among the 5 groups (p = 0.011).
| <120 (n = 199) | 120 to 130 (n = 301) | 130 to 140 (n = 139) | 140 to 150 (n = 221) | ≥150 (n = 135) | p | |
|---|---|---|---|---|---|---|
| Women | 89 (44.7) | 109 (36.2) | 53 (38.1) | 80 (36.2) | 48 (35.6) | 0.301 |
| Asian race | 114 (57.3) | 170 (56.5) | 69 (49.6) | 132 (59.3) | 73 (54.1) | 0.421 |
| Age, yr | 64.2 ± 12.7 | 62.2 ± 13.2 | 61.6 ± 13.8 | 60.5 ± 12.5 | 60.9 ± 12.9 | 0.053 |
Onset-to-randomization time, min |
176.3 ± 56.1 | 183.8 ± 59.2 | 181.1 ± 54.8 | 187.7 ± 59.6 | 190.0 ± 49.8 | 0.245 |
| Baseline NIHSS score | 11 [6–16] | 11 [6–16] | 11 [6–17] | 10 [6.8–15.0] | 12 [8–17] | 0.226 |
| Baseline hematoma volume, cm3 | 9.3 [4.9–16.4] | 10.8 [4.8–19.6] | 10.6 [4.9–19.1] | 9.3 [5.3–16.4] | 13.6 [6.7–24.7] | 0.011 |
| Lobar location of hematoma | 23 (11.6) | 29 (9.7) | 15 (10.9) | 28 (12.9) | 15 (11.2) | 0.858 |
| Intensive treatment group | 182 (91.5) | 262 (87.0) | 47 (33.8) | 5 (2.3) | 2 (1.5) | <0.001 |
- Values are n (%), mean ± standard deviation, or median [interquartile ratio].
- NIHSS = National Institutes of Health Stroke Scale.
At 90 days, mRS was assessed in 959 subjects. Score was 4 to 6 in 365 subjects (38.1%), and 66 (6.9%) had died. No linear association among the 5 SBP groups were observed with mRS 4 to 6 (p = 0.648) or death (p = 0.779). All analyses designated the 120- to 130-mmHg group as the reference. After multivariable adjustment, the 140- to 150-mmHg group showed a higher risk of mRS 4 to 6 than the 120- to 130-mmHg group (odds ratio [OR], 1.62; 95% confidence interval [CI], 1.02–2.58; Table 2; Fig 2). Multivariable analysis did not reveal any significant difference in mortality among the SBP groups.
| <120 | 120 to 130 | 130 to 140 | 140 to 150 | ≥150 | pc | |
|---|---|---|---|---|---|---|
| mRS 4 to 6 at 90 days | 72/192 (37.5) | 105/292 (36.0) | 56/131 (42.8) | 83/215 (38.6) | 49/129 (38.0) | 0.648 |
| Unadjusted OR | 1.07 (0.73, 1.56) | REF | 1.33 (0.87, 2.02) | 1.12 (0.78, 1.61) | 1.09 (0.71, 1.67) | |
| Adjusted ORa | 1.00 (0.62, 1.61) | REF | 1.41 (0.83, 2.41) | 1.62 (1.02, 2.58) | 0.93 (0.55, 1.64) | |
| Death at 90 days | 12/192 (6.3) | 19/292 (6.5) | 12/131 (9.2) | 14/215 (6.5) | 9/129 (7.0) | 0.779 |
| Unadjusted OR | 0.96 (0.44, 2.00) | REF | 1.45 (0.66, 3.05) | 1.00 (0.48, 2.03) | 1.08 (0.45, 2.39) | |
| Adjusted ORa | 0.84 (0.35, 1.98) | REF | 1.27 (0.51, 3.07) | 1.30 (0.53, 3.11) | 1.19 (0.43, 3.08) | |
| Hematoma expansion | 31/184 (16.9) | 38/278 (13.7) | 28/131 (21.4) | 38/205 (18.5) | 34/129 (26.4) | 0.013 |
| Unadjusted OR | 1.28 (0.76, 2.14) | REF | 1.72 (0.99, 2.94) | 1.44 (0.88, 2.35) | 2.26 (1.34, 3.81) | |
| Adjusted ORa | 1.35 (0.77, 2.37) | REF | 1.57 (0.86, 2.84) | 1.80 (1.05, 3.09) | 1.98 (1.12, 3.51) | |
| Cardiorenal AEs | 20/199 (10.1) [R 4, C 17]b |
35/301 (11.6) [R 13, C 24] |
14/139 (10.1) [R 4, C 10] |
12/221 (5.4) [R 3, C 10] |
8/135 (5.9) [R 3, C 6] |
0.019 |
| Unadjusted OR | 0.85 (0.47, 1.50) | REF | 0.85 (0.43, 1.61) | 0.44 (0.21, 0.84) | 0.48 (0.20, 1.01) | |
| Adjusted ORa | 0.86 (0.46, 1.57) | REF | 0.72 (0.35, 1.43) | 0.43 (0.19, 0.88) | 0.44 (0.18, 0.96) |
- Values are n/total (%) or odds ratio [OR] (95% confidence interval [CI]).
- a Adjusted for sex, Asian race, age (quartile), onset-to-randomization time (quartile), baseline National Institutes of Health Stroke Scale (quartile), baseline hematoma volume (quartile), and lobar hematoma.
- b Number of subjects with renal (R) and cardiac (C) adverse events (AEs). Five subjects had both AEs.
- c p for linear trends by the Cochran–Armitage trend test.
- mRS = modified Rankin scale.
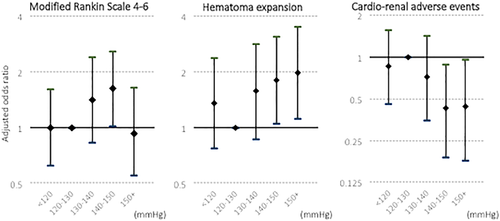
Follow-up CT was examined in 927 subjects, of whom 169 (18.2%) experienced HE. Subjects with HE more commonly showed mRS 4 to 6 than those without HE overall (65.6% versus 29.4%; p < 0.001), as well as in all the 5 SBP categories (Table 3). HE increased linearly with the SBP groups (p = 0.013). After multivariable adjustment, the 140- to 150-mmHg group (OR, 1.80; 95% CI, 1.05–3.09) and > 150-mmHg group (OR, 1.98; 95% CI, 1.12–3.51) exhibited a higher risk of HE than the 120- to 130-mmHg group (Table 2; Fig 2).
| SBP (mmHg) | With Hematoma Expansion (HE) | Without HE | p | With Cardiorenal AEs | Without Cardiorenal AEs | p |
|---|---|---|---|---|---|---|
| <120 | 19 (63.3) | 43 (29.1) | <0.001 | 46 (67.7) | 247 (35.3) | <0.001 |
| 120 to 130 | 29 (76.3) | 61 (26.4) | <0.001 | 22 (62.9) | 83 (32.3) | <0.001 |
| 130 to 140 | 19 (73.1) | 32 (32.7) | <0.001 | 13 (100) | 43 (36.4) | <0.001 |
| 140 to 150 | 22 (57.9) | 51 (31.5) | 0.002 | 7 (53.9) | 76 (37.6) | 0.149 |
| ≥150 | 18 (58.1) | 28 (30.1) | 0.005 | 7 (58.3) | 45 (37.2) | 0.470 |
| Total | 107 (65.6) | 215 (29.4) | <0.001 | 4 (50.0) | 308 (35.3) | <0.001 |
- N (%).
- AEs = adverse events.
Renal and cardiac AEs within 7 days were assessed for all 995 subjects. Of 65 subjects with any renal AEs, 27 had moderate or more-severe AEs, including acute renal failure/impairment in 17, increased serum creatinine in 2, urinary retention in 2, and others in 6. Of 99 subjects with any cardiac AEs, 67 had moderate or more-severe AEs, including cardiac arrest in 10, atrial fibrillation in 8, other arrythmias in 11, hypertension and hypotension in 6 each (symptomatic hyper-/hypotension or too high/low BP levels requiring emergent medical care), heart failure in 5, chest pain in 4, acute myocardial infarction in 2, increase in troponin level in 3, circulatory collapse in 2, and others in 10. Details of AEs are described elsewhere.8 Five subjects had both renal and cardiac AEs. Thus, 89 subjects (8.9%) had moderate or more-severe renal or cardiac AEs.
Subjects with cardiorenal AEs more commonly showed mRS 4 to 6 than those without AEs overall (50.0% versus 35.3%; p < 0.001), as well as in the 3 SBP categories <140 mmHg (Table 3). Separate analysis of renal AEs alone (59.3% versus 37.5%; p = 0.021) and cardiac AEs alone (66.2% versus 36.0%; p < 0.001) also showed positive relationship between AEs and mRS 4 to 6. Cardiorenal AEs increased linearly with the SBP groups (p = 0.019). After multivariable adjustment, subjects with SBP 140 to 150 mmHg (OR, 0.43; 95% CI, 0.19–0.88) and > 150 mmHg (OR, 0.44; 95% CI, 0.18–0.96) showed a lower risk of cardiorenal AEs than subjects with SBP 120 to 130 mmHg (Table 2; Fig 2).
Multivariable analysis for outcomes were repeated by further adding treatment assignment as an adjusting variable. The 140- to 150-mmHg group showed a higher risk of mRS 4 to 6 than the 120- to 130-mmHg group (OR, 2.08; 95% CI, 1.07–4.08; Supplementary Table 1). Otherwise, there were no significant differences in any outcomes among the SBP groups.
Analysis Using the Reduction of Average Hourly Minimum SBP From the Initial SBP
Frequency of outcomes, as well as their multivariable association with the absolute SBP reduction, are shown in Table 4. There was no linear relationship (p = 0.471) or significant multivariable association between the 5 groups in the 18-mmHg strata and mRS 4 to 6. There was a downward linear relationship between SBP reduction and death (p = 0.034); after multivariable adjustment, the largest reduction group showed a lower risk of death than the smallest reduction group (OR, 0.25; 95% CI, 0.07–0.74). HE decreased linearly (p < 0.001) and cardiorenal AEs increased linearly (p = 0.021) with SBP reduction. After multivariable adjustment, the larger 3 reduction groups showed a lower risk of HE and the largest reduction group showed a higher risk of cardiorenal AEs than the smallest reduction group.
| <40 mmHg | 40 to 58 mmHg | 58 to 76 mmHg | 76 to 94 mmHg | ≥94 mmHg | pb | |
|---|---|---|---|---|---|---|
| mRS 4 to 6 at 90 days | 72/164 (43.9) | 64/180 (35.6) | 91/253 (36.0) | 77/200 (38.5) | 61/161 (37.9) | 0.471 |
| Adjusted OR | REF | 0.74 (0.43, 1.26) | 0.90 (0.55, 1.48) | 0.87 (0.52, 1.47) | 0.79 (0.45, 1.38) | |
| Death at 90 days | 17/164 (10.4) | 15/180 (8.3) | 14/253 (5.5) | 12/200 (6.0) | 8/161 (5.0) | 0.034 |
| Adjusted OR | REF | 1.13 (0.48, 2.69) | 0.55 (0.23, 1.32) | 0.49 (0.20, 1.21) | 0.25 (0.07, 0.74) | |
| Hematoma expansion | 45/158 (28.5) | 45/176 (25.6) | 36/247 (14.6) | 24/190 (12.6) | 19/155 (12.3) | <0.001 |
| Adjusted OR | REF | 0.90 (0.52, 1.56) | 0.48 (0.28, 0.83) | 0.36 (0.19, 0.64) | 0.36 (0.19, 0.68) | |
| Cardiorenal AE | 14/169 (8.3) [R 1, C 13]a |
12/187 (6.4) [R 4, C 10] |
19/263 (7.2) [R 9, C 16] |
19/208 (9.1) [R 6, C 14] |
25/167 (15.0) [R 7, C 14] |
0.021 |
| Adjusted OR | REF | 0.95 (0.41, 2.19) | 1.09 (0.52, 2.34) | 1.22 (0.58, 2.63) | 2.11 (1.01, 4.55) |
- Values are n/total (%) or odds ratio [OR] (95% confidence interval [CI]).
- Adjusted for sex, Asian race, age (quartile), onset-to-randomization time (quartile), baseline National Institutes of Health Stroke Scale (quartile), baseline hematoma volume (quartile), and lobar hematoma.
- a Number of subjects with renal (R) and cardiac (C) adverse events (AE). Five subjects had both AEs.
- b p for linear trends by the Cochran–Armitage trend test.
- mRS = modified Rankin scale.
Frequency of outcomes, as well as their multivariable association with the relative SBP reduction, are shown in Supplementary Table 2. There was no significant multivariable association between the 5 groups in 6% strata and mRS 4 to 6. After multivariable adjustment, the largest reduction group showed a lower risk of death than the smallest reduction group (OR, 0.35; 95% CI, 0.13–0.87). HE decreased linearly (p < 0.001) and cardiorenal AEs increased linearly (p = 0.024) with SBP reduction. After multivariable adjustment, the larger 3 reduction groups showed a lower risk of HE than the smallest reduction group.
Analysis Using Average Hourly Mean SBP Levels
Distribution of average mean SBP between 2 and 24 hours is shown in Figure 1 (right). Median average mean SBP was 137.9 mmHg (IQR, 128.1–152.6 mmHg). Subjects were divided into the following 5 groups based on SBP: <125 (n = 134); 125 to 135 (n = 326); 135 to 145 (n = 134); 145 to 155 (n = 215); and ≥ 155 mmHg (n = 186).
Baseline characteristics of subjects in the 5 groups were similar to those in the 5 groups by average minimum SBP, although intergroup differences were clearer (Supplementary Table 3).
Results of multivariable analysis on outcomes are demonstrated in Table 5. No significant associations were apparent between groups depending on SBP and mRS 4 to 6 at 90 days. Associations between groups and secondary outcomes resembled those in the analyses using average minimum SBP.
| <125 | 125 to 135 | 135 to 145 | 145 to 155 | ≥155 | pb | |
|---|---|---|---|---|---|---|
| mRS 4 to 6 at 90 days | 43/133 (32.3) | 120/311 (38.6) | 54/130 (41.5) | 72/208 (34.6) | 76/177 (42.9) | 0.244 |
| Adjusted OR | 0.92 (0.53, 1.56) | REF | 1.14 (0.68, 1.91) | 1.21 (0.76, 1.92) | 1.24 (0.78, 1.98) | |
| Death at 90 days | 5/133 (3.8) | 22/311 (7.1) | 12/130 (9.2) | 16/208 (7.7) | 11/177 (6.2) | 0.508 |
| Adjusted OR | 0.62 (0.18, 1.83) | REF | 1.14 (0.47, 2.66) | 1.65 (0.74, 3.64) | 0.93 (0.36, 2.25) | |
| Hematoma expansion | 18/128 (14.1) | 42/302 (13.9) | 27/122 (22.1) | 39/199 (19.6) | 43/176 (24.4) | 0.003 |
| Adjusted OR | 1.04 (0.53, 1.97) | REF | 1.85 (1.02, 3.34) | 1.64 (0.97, 2.78) | 2.07 (1.23, 3.50) | |
| Cardiorenal AE | 11/134 (8.2) [R 2, C 10]a |
38/326 (11.7) [R 11, C 29] |
16/134 (11.9) [R 8, C 8] |
14/215 (6.5) [R 2, C 12] |
10/186 (5.4) [R 4, C 8] |
0.037 |
| Adjusted OR | 0.88 (0.40, 1.80) | REF | 0.94 (0.47, 1.78) | 0.56 (0.28, 1.09) | 0.39 (0.17, 0.80) |
- Values are n/total (%) or odds ratio [OR] (95% confidence interval [CI]).
- Adjusted for sex, Asian race, age (quartile), onset-to-randomization time (quartile), baseline National Institutes of Health Stroke Scale (quartile), baseline hematoma volume (quartile), and lobar hematoma.
- aNumber of subjects with renal (R) and cardiac (C) adverse events (AE). Five subjects had both AEs.
- bp for linear trends by the Cochran–Armitage trend test.
- mRS = modified Rankin scale.
Discussion
In the present exploratory analyses of the ATACH-2 data, we sought to clarify the association between SBP achieved in the initial 24 hours of death and severe disability (mRS 4–6) at 90 days and the relevant radiological and safety outcomes in acute ICH patients. The major new findings were first that mRS 4 to 6 was less common when the average minimum SBP achieved was 120 to 130 mmHg than when the level was 140 to 150 mmHg, although the association was modest and not reproduced when analyzed using average mean SBP levels. Second, risk of HE, that was positively associated with mRS 4 to 6, showed a linear increase according to increasing average minimum SBP achieved from 120 to 130 mmHg. Third, cardiorenal AEs within 7 days, that was also positively associated with mRS 4 to 6, were less common among subjects with SBP ≥140 mmHg than among subjects with 120 to 130 mmHg. Thus, any beneficial effects of lowering SBP to 120 to 130 mmHg on clinical outcomes after ICH by suppressing HE seemed to be somewhat offset by cardiorenal complications.
In the present analysis using achieved SBP levels, the positive association of SBP levels with mRS 4 to 6 at 90 days was identified only between the average minimum SBP of the 120- to 130-mmHg group and 140- to 150-mmHg group. The association remained positive after further adjustment by the treatment assignment. Thus, the largest proportion (53%) of the intensive treatment group, which targeted the minimum SBP to less than 140 mmHg, showed better outcome than the largest proportion (43%) of the standard treatment group, which targeted the minimum SBP to 140 to 160 mmHg. This aligns with the main results of the INTERACT2,6 although the difference was modest.
HE during the initial hours to days is an established predictor of poor clinical outcomes after ICH.11, 12 Deeper and more-rapid lowering of SBP was reported to provide greater attenuation of HE.5, 13-15 Because intravenous nicardipine, a potent antihypertensive agent, was uniformly chosen for BP lowering in the ATACH-2 (as opposed to INTERACT2, where no restrictions were placed on antihypertensive agents, including oral ones), we anticipated that the ATACH-2 would show clearer protective results on suppression of HE and thus better clinical outcomes.16 Therefore, the ATACH-II showed >10 mmHg lower levels of achieved SBP during the initial 24 hours compared to the INTERACT2, as well as more-rapid emergency room arrival. The hourly minimum SBP an hour after randomization was ≈140 mmHg in the ATACH-2, and hourly SBP was ≈150 mmHg in the INTERACT2.6, 8 Such intensive lowering of SBP resulted in marginally greater attenuation of HE in the ATACH-2, but was not associated with decreases in mRS 4 to 6. A similar discrepancy was observed between the INTERACT1 and INTERACT2.6, 17 Intensive lowering of SBP also might introduce triggers that offset the favorable effects of suppressing HE on outcomes.
A sudden decrease in BP, particularly brought about by systemic arterial dilatation, can cause renal ischemic injury.18 In the pilot phase of the ATACH, involving 60 ICH subjects with acute infusion of intravenous nicardipine within 6 hours of symptom onset, 5 (9%) developed acute renal injury (stage I for all) according to the Acute Kidney Injury Network criteria and showed higher incidences of neurological deterioration and symptomatic HE than other 55 subjects.19 In the present study, seven-tenths of renal AEs were associated with renal failure, renal impairment, and increased serum creatinine, all suggesting acute kidney injury possibly attributed to acute decrease in BP. Prerenal kidney injury usually does not accompany severe morphological renal damage and can be normalized by appropriate fluid replacement.18, 20 Careful monitoring of kidney function and care for kidney dysfunction are required after acute intensive BP lowering. Cardiac AEs were also positively associated with mRS 4 to 6 in this study. Such cardiorenal accidents might weaken the beneficial effects of BP lowering on ICH outcomes. When assessing the association between cardiorenal AEs and mRS 4 to 6 at 90 days at different SBP categories, subjects with mRS 4 to 6 were more common in subjects with the AEs than those without the AEs, especially in the lower SBP categories.
Analyses using the absolute and relative SBP reductions generally reproduced the results of those using the achieved SBP levels. Frequency of mRS 4 to 6 was not influenced by extent of SBP reduction. Lowering SBP was relatively linearly associated with both decrease in HE and increase in cardiorenal AEs. Interestingly, the effect of HE suppression was observed in a smaller extent of SBP reduction than increase in cardiorenal AEs.
The latest versions of guidelines in Europe and the United States were published before publication of the main results from the ATACH-2 and, accordingly, does not reflect its results.22, 23 In the revised Guidelines for the Management of Hypertension from the Japanese Society of Hypertension to be published in 2019, the plan is to recommend that “acute lowering of SBP to 140mmHg can be considered for patients with acute ICH, though renal dysfunction accompanied with antihypertensive therapy requires special attention” (personal communication) according to the results of the ATACH-2. Given that prerenal acute kidney injuries are treatable, prompt care for such hypotension-associated events may increase the safety of intensively lowering SBP.
All of the above guidelines recommended acute intensive lowering of SBP to 140 mmHg in cases of acute ICH.21, 22 Recent meta-analyses, including the ATACH-2, however, have expressed skepticism regarding the efficacy of intensive lowering.9, 10 We should be mindful of the fact that target levels of intensive and standard SBP lowering differed between the ATACH-2 and INTERACT2. In the INTERACT2, lowering SBP to 130 to 139 mmHg was likely to maximally decrease the risk of mRS 3 to 6.23 In the multicenter observational SAMURAI (Stroke Acute Management with Urgent Risk-factor Assessment and Improvement)-ICH study, where SBP was lowered to <160 mmHg using intravenous nicardipine for 211 acute ICH patients, the achieved SBP level of <130 mmHg during the initial 24 hours was independently and inversely associated with mRS 4 to 6, as compared with the level ≥ 145 mmHg.5, 24 The present exploratory analyses indicated that the achieved average hourly minimum SBP of 120 to 130 mmHg showed a gentle peak for better clinical outcomes. Taking all findings together, mean SBP around 130 mmHg might be the optimal goal during the initial 24 hours in acute ICH care. The ongoing analysis of combined data from the INTERACT2 and ATACH-2 using the present methods may provide clearer levels for goal SBP.
The limitations of the present study included the fact that subjects were limited to those meeting the inclusion criteria for the ATACH-2. Thus, some typical ICH patients with more-severe symptoms who need intensive therapy were excluded, including those with huge hematoma ≥60cm3 in volume and those with infratentorial hemorrhage. Second, the present results might not be generalizable to patients with antihypertensive treatments other than intravenous nicardipine.
Acknowledgment
Supported by grants (U01-NS062091 to Qureshi; U01-NS061861 and U01-NS059041 to Palesch) from the National Institute of Neurological Disorders and Stroke and by a grant (H28-4-1 to Toyoda) from the Intramural Research Fund for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center.
Author Contributions
K.T., M.K., H.Y., Y.Y.P., S.S., and A.I.Q. contributed to the conception and design of the study. K.T., M.K., L.F., Y.W., N.S., T.H., C.Y.H., R.I., S.S., M.F., T.S., B.-W.Y., and D.F.H. contributed to the acquisition and analysis of data. K.T., M.K., and S.S. drafted the manuscript.
Potential Conflicts of Interest
Nothing to report.




