Facioscapulohumeral Dystrophy in Childhood: A Nationwide Natural History Study
Abstract
Objective
Facioscapulohumeral dystrophy (FSHD) is one of the most frequent heritable muscular dystrophies, with a large variety in age at onset and disease severity. The natural history and molecular characteristics of FSHD in childhood are incompletely understood. Our objective is to clinically and genetically characterize FSHD in childhood.
Methods
We performed a nationwide, single-investigator, natural history study on FSHD in childhood.
Results
Multiple-source recruitment resulted in 32 patients with FSHD (0–17 years), leading to an estimated prevalence of 1 in 100,000 children in The Netherlands. This series of 32 children with FSHD revealed a heterogeneous phenotype and genotype in childhood. The phenotypic hallmarks of FSHD in childhood are: facial weakness with normal or only mildly affected motor performance, decreased functional exercise capacity (6-minute walk test), lumbar hyperlordosis, and increased echo intensity on muscle ultrasonography. In addition, pain and fatigue were frequent and patients experienced a lower quality of life compared to healthy peers. In contrast to the literature on early-onset FSHD, systemic features such as hearing loss and retinal and cardiac abnormalities were infrequent and subclinical, and epilepsy and intellectual disability were absent. Genotypically, patients had a mean D4Z4 repeat array of 5 units (range, 2–9), and 14% of the mutations were de novo.
Interpretation
FSHD in childhood is more prevalent than previously known and the genotype resembles classic FSHD. Importantly, FSHD mainly affects functional exercise capacity and quality of life in children. As such, these results are paramount for counseling, clinical management, and stratification in clinical research. Ann Neurol 2018;84:635–645
Facioscapulohumeral dystrophy (FSHD) is one of the most frequent heritable muscular dystrophies and typically affects the facial, scapulohumeral, tibial, and axial muscles.1, 2 The most frequent cause of FSHD, which is currently the only reported cause of FSHD in childhood, is partial loss of D4Z4 macrosatellite repeats in the subtelomere of chromosome 4q (FSHD type 1 [FSHD1]; OMIM 158900). FSHD1 patients have only 1 to 10 of these D4Z4 repeats, whereas healthy individuals have 8 to 100 repeats.3 Loss of D4Z4 repeats results in partial D4Z4 chromatin relaxation and derepression of the DUX4 in skeletal muscles,4 a germ-line and cleavage stage transcription factor encoded by a conserved open reading frame within the D4Z4 repeat. Presence of DUX4 in skeletal muscle activates a series of transcriptional programs, eventually leading to muscle cell death.4 In the rare form, FSHD2, D4Z4 chromatin relaxation and DUX4 derepression in skeletal muscle are caused by mutations in the chromatin regulator, SMCHD1, or rarely DNMT3B.5, 6 The FSHD phenotype encompasses a broad spectrum of severity ranging from nonpenetrant mutation carriers to severely affected patients.
Age at onset of FSHD varies from infancy to late adulthood. Whereas most patients present in the second or third decade of life, up to 21% of patients are estimated to present in childhood.7 Initially, early-onset FSHD was defined by (1) facial weakness before the age of 5 years and (2) scapulohumeral weakness before the age of 10 years.8 In literature, early-onset FSHD is often described as a severe subtype associated with one to three D4Z4 repeats, severe muscle weakness, and frequent systemic complications, including epilepsy, hearing difficulties, retinal abnormalities (Coats’ syndrome), intellectual disability, and cardiac arrhythmias.8-14
Currently, FSHD in childhood is often perceived as synonymous to early-onset FSHD. Therefore, FSHD in childhood is frequently described as being a severely affected subgroup. However, this is solely based on case reports and case series, and, in practice, we also encounter children with milder phenotypes in our tertiary referral center. The full spectrum of FSHD in childhood is therefore probably not known. Furthermore, data on the prevalence, natural history, prognostic markers, and clinical management of FSHD in childhood are limited. These data are critical for a better and focused understanding of childhood FSHD, optimal patient care, and improved clinical trial readiness.15 We therefore instigated the first population-based, nationwide, single-center study on childhood FSHD.
The objectives of this study are to (1) clinically and genetically characterize FSHD in childhood and (2) estimate the prevalence of FSHD in childhood in The Netherlands.
Patients and Methods
Patients and Design
A nationwide, prospective, cross-sectional study was performed. Eligible patients had clinically suspected FSHD, defined as clinical weakness of the facial and/or upper arm muscles for which the patient had sought medical attention with exclusion of other diagnoses. Patients were 17 years or younger and resided in The Netherlands. Patients were recruited by (1) the database of our tertiary referral center, which was established in 1986; (2) neurologists/pediatricians from all neuropediatric clinics in The Netherlands; (3) the Department of Clinical Genetics of the Leiden University Medical Centre, the single reference center for genetic testing of FSHD in The Netherlands; and (4) by social media groups focusing on FSHD. This extensive recruitment ensured a high degree of inclusion (protocol published previously16). Patients were recruited from December 2015 until August 2017. Clinical and molecular data were collected prospectively. This study protocol has been approved by the Medical Review Ethics Committee region Arnhem-Nijmegen (NL53213.091.15) and is in accord with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent from participants aged 12 to 18 years and from parents/legal guardians of all participants was obtained.
Mutation Analysis
Peripheral blood mononuclear cells were collected from patients and their biological parents, if available, and high-molecular-weight DNA and RNA was isolated. Sizing of the D4Z4 repeats on chromosomes 4 and 10 was performed by pulsed field gel electrophoresis (PFGE), followed by Southern blotting using probe p13E-11. Haplotype analysis was done by sequential hybridization of PFGE Southern blots with probes A and B in combination with PCR-based simple-sequence length polymorphism analysis.3, 17 We also established the methylation of the D4Z4 repeat at the FseI restriction site in the proximal unit of the D4Z4 array.6, 18 If appropriate, we tested for mutations in SMCHD1 or DNMT3B by PCR amplification of all coding exons followed by Sanger sequencing.6 Methylation values are expressed as the Delta1 score, that is, the observed methylation minus the predicted methylation based on the D4Z4 repeat size.19 Delay in diagnosing FSHD was defined as the time course between first symptoms/first medical assessment and the genetically confirmed diagnosis of FSHD.
Clinical Assessments
Clinical outcome measures were structured according to the format of the International Classification of Functioning, Disability and Health for children and youth (ICF-CY20; Supporting Information Appendix I) and all measures were performed by one team (R.J.M.G., neurologist in training and M.J., pediatric physiotherapist).
The clinical phenotype was assessed by manual muscle force testing (trapezius, deltoid, biceps, finger flexors, quadriceps femoris, tibialis anterior, and gastrocnemicus muscle)21 endurance by means of the 6-minute walk test22-24 with calculation of the percent change in velocity of the first- and last-minute distance.25 Patients were graded by the FSHD clinical score26 and the age-adjusted clinical severity scale.27, 28 Both parents were assessed clinically, and the clinical severity scale27 was determined. We defined parents with a molecular diagnosis of FSHD as affected, asymptomatic (no reported symptoms and only mild signs on clinical examination), or as nonpenetrant (no reported symptoms and no signs on clinical examination). Muscle functions were tested by means of the Motor Function Measure29, 30 and the shoulder dimension of the Performance of the Upper Limb (PUL) module.31
Quality of life and daily functioning were assessed using educational level, pain score, the Kidscreen questionnaire,32 and the NeuroQol fatigue domain.33 Normal values of the Kidscreen and the NeuroQoL were provided by the investigators,32, 33 and results are expressed as standard deviations (SDs) from the mean.
Ophthalmologic examinations included visual acuity using a Snellen chart, clinical examination by an ophthalmologist, fundusphotography, and optical coherence tomography (OCT), as well as OCT angiography for specific examination of the fovea.34 A tone and speech audiometry was performed to detect (sub)clinical hearing loss and an electrocardiogram (ECG) to detect cardiac abnormalities.
Muscle Ultrasound Analysis
All ultrasound examinations were performed on a MyLab Twice muscle ultrasound (MUS) system (Esaote, Genoa, Italy), using a 3- to 13-MHz broadband linear transducer (LA533). Quantative muscle ultrasound (QMUS) measurements etc. were performed to determine echo intensity of the masseter, trapezius, biceps brachii, rectus abdominis, rectus femoris, gastrocnemius, and tibialis anterior muscles, bilaterally. Choice of muscles was based on the frequency of involvement described in FSHD35 and the availability of US normal values (therefore no facial muscles typically affected in FSHD were imaged). A fixed scanning protocol36 was used and the captured images were analyzed for echo intensity by means of computer-assisted grayscale histogram analysis, using custom software (QUMIA). Echo intensity was compared to muscle specific reference values and expressed as z-score, a robust measure for qMUS analysis analysis.37
Statistical Analysis
Statistical analysis was performed using SPSS (version 22.0; SPSS, Inc., Chicago, IL)38 and Prism software (version 5; GraphPad Software Inc., San Diego, CA). Parametric variables are expressed as mean ± SD. Nonparametric data are expressed as median [interquartile range]. Student t and Mann-Whitney U tests were used for comparing the numerical data. Results of the NeuroQol fatigue scale, 6-minute walk test, and Kidscreen were expressed as the number of SDs from the mean (z-score). A p value < 0.05 was considered statistically significant.
To investigate possible differences between early-onset FSHD and classic FSHD, subgroup analysis were done on three subgroups: (1) patients with “early-onset FSHD” based on the following criteria: (i) signs or symptoms of facial weakness before the age of 5 years and (ii) signs or symptoms of scapular weakness before the age of 10 years8; (2) patients with “classic FSHD” (eg, those with onset after the age of 10 years); and (3) patients who were too young to be classified in one of the above categories.
Results
Demographics
A total of 38 children were identified as possible participants: 28 by the national genetic reference center database and 10 children with clinically suspected FSHD by other recruitment sources. Of these 10 children with clinically suspected FSHD, FSHD was molecularly confirmed in 4 children. In 3 children, a different diagnosis was found (mitochondrial myopathy, celiac disease, and chronic fatigue syndrome), and the other 3 had a physiological asymmetry in facial and/or scapular muscles without the underlying genetic defect known in their families. A total of 32 children with genetically confirmed FSHD were identified (Fig 1). The total number of children aged 0 to 18 years in The Netherlands is 3.416.581 (Statistics Netherlands39), leading to a lower-bound prevalence estimate of 1 in 100,000. Of the 32 children, 28 participated in the study (19 at the study location, 3 at home, and 6 with medical file review). Four patients did not participate, but their age at onset and genetic characteristics were known: 1 patient refused participation because of the parents’ fear for the emotional burden of the genetic results; the other 3 could not be identified or traced (Fig 1). Participants were aged 2 to 17 years with a mean age at examination of 11.5 years and a mean age at onset of 6.7 years. Clinical and genetic characteristics can be found in Table 1. Creatine kinase was moderately increased with a mean of 180U/l; levels ranged from 37 to 500U/l (normal-to-moderate increase).
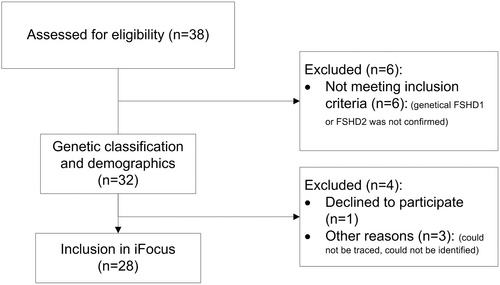
Flow diagram of patient recruitment and inclusion. FSHD = facioscapulohumeral dystrophy.
| Demographics | N | Mean | Range | SD |
|---|---|---|---|---|
| Age at examination (y) | 32 | 12 | 4 to 17 | 5 |
| Male sex, no. (%) | 14 (44) | |||
| D4Z4 repeat arrays, mean (range, SD) | 32 | 5.2 | 2 to 10 | 2.0 |
| Methylation score, mean (range, SD) | 12 | –6.18 | –13 to 5 | 5.2 |
| De novo mutations, no./total no. (%) | 3/22 (14) | |||
| Mosaic inheritance, no./total no. (%) | 0/22 (0) | |||
| SMCHD1 mutation | 1/22 | |||
| Motor Functioning | ||||
| Facial weaknessa | 21/25 | |||
| Scapular weakness (PUL)b | 4/12 | |||
| Motor function measure | 18 | 99.4% | 96% to 100% | 1.1 |
| MRC sum score (0–70) | 12 | 68.1 | 54 to 70 | |
| Six-minute walk test (number of SDs) | 12 | –2.2 | –5.24 to –0.9 | |
| Clinical severity scale (CSS, 0–10) | 20 | 2.5 | 0 to 6 | 1.5 |
| Age-corrected CSS (0–2,000) | 20 | 448 | 0 to 1,200 | 306 |
| FSHD evaluation score (0–15) | 20 | 2.3 | 0 to 6 | 1.5 |
| Systemic Features | Measured | Complaints | Investigational Findings |
|---|---|---|---|
| Hearing loss | 29 | 0 | 3/19 high-frequency loss on audiometry (16%) |
| Vision loss | 22 | 0 | 5/9 retinopathy on fundoscopy (56%) |
| Epilepsy, no./total no. | 27 | 0 | 0/2 abnormalities on EEG |
| Intellectual disability | 27 | 0c | |
| Cardiac abnormalities | 22 | 1 | 4/22 abnormalities on ECG |
| Lumbar hyperlordosis | 24 | 3d | 9/24e |
| Assisted ventilation | 32 | 0 | 1/9 decreased spirometry |
| Swallowing difficulties | 22 | 1 | 0/12 swallowing abnormalities |
| Dysarthria | 22 | 5 | 3/22 mild dysarthria on speech language examination |
- a Based on one or more points on the facial weakness domain of the FSHD clinical score.
- b Performance of the upper limb shoulder module.31
- c Defined by school level; none of the patients attended specialized education.
- d Based on complaints of upper or lower back pain.
- e Based on clinical examination of the spine.
- CSS = Clinical Severity Score; ECG = electrocardiogram; EEG = electroencephalogram; FSHD = facioscapulohumeral dystrophy; MRC = Medical Research Council; PUL = Performance of Upper Limb; SD = standard deviation.
Genetic Characteristics
Average diagnostic delay of FSHD in childhood was 3.1 years (range, 0–9). Six patients did not have a genetic diagnosis before enrollment in the study. The reason for abstaining from earlier genetic testing was the possible emotional burden for the child combined with the lack of curative treatment. In contrast, 5 patients from three families had elected presymptomatic testing after genetic counseling.
Of the 28 patients, 6 declined venipuncture and in the other 22 patients both the child and parents were genetically tested. In 19 cases (86%), an autosomal-dominant transmission was found, eight paternal and 11 maternal transmissions. In the other 3 cases (14%), the mutation had occurred de novo as a germinal mutation. Somatic mosaicism was not observed. The parents of 3 patients had been diagnosed after their children were diagnosed: 1 was nonpenetrant, and the other 2 were asymptomatic with mild facial and scapular weakness. Mean number of units within the pathogenic D4Z4 repeat was 5.2 (SD, 2; range, 2–10). In 12 children, comprehensive genetic characterization was performed, showing delta1 scores below –10% in 5, but not below the threshold for FSHD2 (–21%). Another patient was diagnosed with FSHD2 and carried a pathogenic SMCHD1 variant. No significant correlation between mean number of units within the pathogenic D4Z4 repeat and disease severity (as measured by the FSHD clinical score, the age-adjusted clinical severity scale, and the motor function measure) or age at onset was observed (Spearman r, all p > 0.05).
Clinical Characteristics
Muscle Functioning
Muscle strength and muscle performance ranged from normal to mildly reduced with a mean MRC sum score of 68 of 70 points (range, 54–70; SD, 2.26), and a Motor Function Measure (MFM) of 99% of 100% (range, 96–100; SD, 1.1). Asymmetry of the Medical Research Council (MRC) score was noted in 4 of 8 patients with a decreased MRC sum score. Lower scores on the MFM were almost exclusively found in the axial and proximal functioning dimension. Four of 12 tested patients had a decreased shoulder-arm function as assessed by the PUL test. These 4 patients were not able to stack or raise cans on different axes, and all showed compensatory strategies such as protraction or elevation of the trunk. In 5 of 12 tested patients, a hamstring shortening was noticed.
Functional exercise capacity, measured by the 6-minute walk test, was significantly below the mean for sex and age (mean, –2.1 SDs; range, –5.2 to 0.9; SD, 1.5; one-sample t test, t = 5.29; p < 0.0001). The patient with the poorest performance on the 6-minute walk test (z-score, –5.2) used a wheelchair for longer distances. Average gait velocity decrease between the first and last minute was 9.6% (82m in the first minute versus 73m in the sixth minute; paired t test, p = 0.002). Mean (range, SD) clinical severity scale was 2.5 of 10 points (0–6, 1.5 points), and mean (range, SD) FSHD evaluation score was 2.3 of 15 points (0–6, 1.5 points).
Muscle US
Quantitative muscle US showed increased echo intensity (ie, mean gray level of muscle images), pointing to muscle fibrosis and/or fatty degeneration in the trapezius, biceps brachii, rectus femoris, tibialis anterior, rectus abdominis, and gastrocnemius on both sides. Echo intensity asymmetries between left and right side were observed in all muscles. The masseter was the only unaffected muscle in all patients. Severity of muscle US abnormalities was heterogenic ranging from completely normal (n = 6) to severely affected (mean MUS echo intensity, 2.5 SDs above average; n = 4). The composite sum score of all measurements combined showed a mean of 1.4 SD above average (Fig 2). All clinically affected muscles had an increased echo intensity, but not all the muscles with increased echo intensity were clinically affected.

Combined ultrasound measurements of 14 participants showing the z-score of quantified echo intensity. The z-score corresponds to the number of standard deviations from the mean score for age and sex and weight using established reference values. This shows that the masseter is similar to reference values whereas all other muscles (trapezius, biceps brachialis, rectus femoris, tibialis anterior, rectus abdominis, and gastrocnemius) have increased echo intensity.
Systemic Features
Mild spinal posture abnormalities were frequent; 5 of 18 patients had an increased lumbar hyperlordosis, and 2 of 18 patients had a scoliotic posture. In none of them was surgical correction required. Three of these 5 patients with lumbar hyperlordosis showed severe abdominal muscle involvement and 2 mild abdominal muscle involvement on muscle US.
Clinically relevant systemic features of FSHD were rare in this cohort: none of the patients or parents reported difficulties of hearing, vision, intellect or development, epilepsy, and heart or pulmonary functioning. We only detected subclinical findings with additional investigations. In 3 of 19 patients, subclinical hearing loss was detected with audiometry; 2 patients had high-tone frequency loss (which is associated with FSHD), and 1 patient had a conductive hearing deficit (which is not associated with FSHD). All patients had a normal visual acuity, and none had abnormalities during ophthalmologic examination. On fundoscopy, retinal abnormalities were frequently observed (6 of 10 patients) consisting of tortuosity of arterial retinal vessels. Capillary leakage or other progressive symptoms of retinal vasculopathy were not found (Fig 3). One patient experienced fatigue and dyspnea at the age of 8 years caused by a patent ductus arteriosus; no other cardiac symptoms were found. ECG revealed minor cardiac abnormalities without clinical consequences: 1 patient with first-degree atrioventricular block; 1 with premature ventricular contractions; and 1 with right-axis deviation. Speech language exam revealed mild dysarthria in 3 patients attributed to decreased lip and facial muscle function and 1 patient with swallowing difficulties. No other gastrointestinal or feeding difficulties were reported. None of the patients had symptoms of decreased respiratory function or needed assisted ventilation.
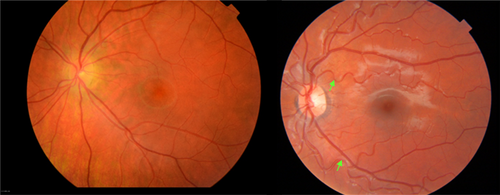
Fundusphotography of 2 independent FSHD patients showing veins (darker and larger in diameter) and arteries (brighter with a central luminance and thinner diameter). (A) Patient 7 with normal appearance of retinal arteries. (B) Patient 9 with severe tortuosity and broader reflex of the retinal arteries (white arrows), but normal appearance of the retinal veins. FSHD = facioscapulohumeral dystrophy. [Color figure can be viewed at www.annalsofneurology.org]
Pain and Daily Life Activities
Pain was a frequent complaint (63%; 15 of 24). Pain during or after prolonged exertion was frequent and located in the lower back, shoulder region, and/or legs. Fatigue was reported in 83% (20 of 24), and the NeuroQol fatigue questionnaire showed that all participants experienced more fatigue than in the general pediatric population (mean, 1.1 SD above average). Quality of life was lower on all domains with a mean SD of 0.9 below average. Specifically, the physical domain was below average (1.5 SD), which corresponds to feelings of exhaustion, feeling unwell, and low energy levels Table 2. All patients attended regular education and participated in after school activities such as sports or music. Parent questionnaires were comparable to the scores of their children.
| N | Mean SD Childa | Mean SD Parenta | Meaning | |
|---|---|---|---|---|
| NeuroQoL 8-item fatigue bank | 10 | 1.1 | 1.2 | Experiencing more fatigue |
| Kidscreen total | 9 | −0.9 | −0.7 | |
| Kidscreen subdomains: | ||||
| Physical well-being | −1.5 | Feeling exhausted, unfit | ||
| Psychological well-being | −1.0 | Dissatisfaction with life | ||
| Autonomy | −0.7 | Feeling restricted | ||
| Parent relation and home life | −0.8 | Feeling alone, overlooked | ||
| Financial resources | −0.7 | Feeling financially disadvantaged | ||
| Social support and peers | −0.8 | Feeling excluded, not accepted | ||
| School environment | −0.7 | Disliking school, not doing well | ||
| Social acceptance | Insufficient number of replies | |||
- a Compared to healthy subjects.
- FSHD = facioscapulohumeral dystrophy; SD = standard deviation.
Early-Onset FSHD Classification
Nine patients fulfilled the criteria for early-onset FSHD,8 17 patients had a classic onset, and 6 patients were too young for classification. Mean (range, SD) current age was 10 years (4–17, 3.5) in the early-onset group and 15 years (9–17, 2.6) in the classic onset group. Early-onset FSHD patients had a shorter repeat length (3.9 versus 5.8 number of units within the pathogenic D4Z4 repeat; Mann-Whitney U, 29.50; p = 0.03), a higher FSHD evaluation score (3.7 versus 1.8; Mann-Whitney U, p = 0.03), and a higher quantitative MUS score (3.2 versus 0.8 mean SDs above average; nonpaired t test, p < 0.005) compared to the classic-onset group. The 6-minute walk test, MFM, and MRC sum score did not differ significantly between the two groups. Individual patient characteristics can be found in Table 3.
| # | Age, Sex | Onset Agea | Onset Type | D4Z4b | Delta Scorec | Hereditary Pattern | MFM (%) | FSHD Score | Ac Scored | SMWT | Systemic Features | MUSe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0, M | pre | na | 4 | Maternal | na | 0 | 0 | na | n | −0.58 | |
| 2 | 2, F | pre | na | 4 | Maternal | na | 0 | 0 | na | n | –0.33 | |
| 3 | 4, M | pre | na | 5 | Paternal | na | 0 | 0 | na | n | ||
| 4 | 4, M | 1 | e | 2 | −3 | Paternal | 100 | 2 | 1,000 | −1.65 | Mild RT hearing loss | |
| 5 | 6, F | 1 | na | 2 | −7 | Paternal | 100 | 2 | 667 | −2.08 | Severe RT | 1.59 |
| 6 | 6, M | 3 | na | 5 | Maternal | 100 | 2 | 667 | n | |||
| 7 | 6, M | pre | na | 7 | Familial | 0 | 0 | u | ||||
| 8 | 8, M | 4 | e | 4 | Maternal | 100 | 3 | 250 | −3.00 | n | ||
| 9 | 8, F | 3 | e | 5 | Maternal | 0 | 0 | ECG1 | ||||
| 10 | 9, F | 1 | e | 8 | Maternal | 96 | 2 | 222 | −3.20 | Mild RT | 2.85 | |
| 11 | 9, F | 8 | c | 4 | −12 | Paternal | 99 | 3 | 667 | n | 0.21 | |
| 12 | 10, M | 7 | e | 2 | −11 | Sporadic | 96 | 6 | 1,200 | −2.71 | LH, severe RT hearing loss | 3.55 |
| 13 | 10, M | 5 | e | 5 | Maternal | 2 | 400 | u | ||||
| 14 | 11, F | 6 | c | 7 | −8 | Maternal | 99 | 3 | 182 | −5.24 | LH, mild RT | 1.42 |
| 15 | 11, M | 1 | e | 5 | −10 | Maternal | 99 | 6 | 364 | −2.50 | LH, S | 2.86 |
| 16 | 11, F | 4 | c | 4 | −2 | Paternal | 100 | 3 | 182 | −1.44 | LH, mild RT | 0.44 |
| 17 | 11, F | 1 | e | 2 | −11 | Sporadic | 99 | 6 | 182 | −0.95 | LH, severe RT | 3.53 |
| 18 | 14, F | 11 | c | 7 | −4 | Maternal | 98 | 2 | 143 | −2.14 | n | –0.29 |
| 19 | 14, M | 12 | c | 6 | Paternal | 100 | 0 | 0 | n | |||
| 20 | 14, F | u | c | 8 | Familial | u | ||||||
| 21 | 15, M | 2 | c | 3 | Maternal | 100 | 3 | 333 | LH | |||
| 22 | 15, F | 8 | c | 5 | Maternal | 100 | 1 | 67 | u | |||
| 23 | 16, F | 15 | c | 6 | −4 | Paternal | 99 | 2 | 313 | −3.17 | S, ECG2 | 0.79 |
| 24 | 16, M | 12 | c | 6 | −14 | Maternal | 100 | 2 | 125 | ECG3 | ||
| 25 | 17, F | 10 | c | 7 | 5 | u | 99 | 1 | 59 | 0.90 | n | 1.05 |
| 26 | 17, M | 13 | c | 5 | u | 100 | 2 | 176 | n | |||
| 27 | 17, F | 16 | c | 7 | −13 | Maternal | 100 | 1 | 118 | −1.60 | Mild RT hearing loss | 1.78 |
| 28 | 17, F | u | c | 10 | Paternal | 100 | 2 | 118 | n | |||
| 29 | 17, M | 3 | e | 5 | Paternal | u | ||||||
| 30 | 17, F | 9 | c | 5 | u | 100 | 3 | n | ||||
| 31 | 17, F | 13 | c | 5 | u | 100 | 1 | 118 | ECG4 | |||
| 32 | 17, M | 15 | c | FSHD2 | Paternal | 100 | 2 | 111 | u |
- a Age at first symptom.
- b Mean number of units within the pathogenic D4Z4 repeat.
- c The observed methylation minus the predicted methylation based on the D4Z4 repeat size.
- d The age-adjusted clinical severity scale.
- e Mean z-score of the echo intensity per muscle.
- # = case number.
- Ac = age corrected; c = classic-onset FSHD; e = early-onset FSHD; ECG1 = patent ductus arteriosus; ECG2 = right-axis deviation; ECG3 = first degree AV-block; ECG4 = sporadic premature ventricular contractions; F = female; FSHD = facioscapulohumeral dystrophy; LH = mild lumbar hyperlordosis posture; M = male; MFM = Motor Function Measure; n = normal/no abnormalities; na = not applicable; pre = presymptomatic; RT = retinal tortuosity; S = scoliotic posture; SMWT = six-minute walk test; u = unknown.
Discussion
This study shows that the clinical spectrum of FSHD in childhood is more heterogeneous than previously reported. In this nationwide study with the largest cohort to date, FSHD in childhood is characterized by facial weakness with otherwise normal muscle strength and performance, limited functional exercise capacity, lumbar hyperlordosis, a decreased quality of physical well-being, and the presence of fatigue and pain. Systemic features are rarely reported. Children with FSHD attend regular schools and extracurricular activities. In general, these findings show that in children, the complete spectrum of classic FSHD is observed instead of the very severe phenotype of early-onset FSHD.
We found a lower-bound estimated prevalence of FSHD in childhood of 1 per 100,000. This is markedly higher than the previously reported 0.29 per 100,000.40 The higher prevalence is likely to be caused by active recruitment resulting in inclusion of the mildly affected children who were not included in earlier prevalence studies. Hence, FSHD is a relatively frequent muscular dystrophy in childhood, approaching the prevalence of myotonic dystrophy in children (1.41 per 100,000). Consequently, it should be considered in children with facial weakness, axial weakness with spinal deformities, and/or scapulohumeral dysfunction. As such, it is an important differential diagnosis of congenital myopathies and congenital myasthenic syndromes. Total FSHD prevalence estimates in literature are, on average, 5 per 100,000,2 leading to an estimated 20% who have a childhood onset.
Genetic characteristics in our cohort were similar to genetic characteristics reported in the adult population: both regarding the repeat size (mean 5.3 units in our cohort versus 5.8 units in an adult white FSHD population41) and frequency of FSHD2 (3.7% versus 5%6). Additionally, whereas the frequency of de novo mutations tends to be higher in early-onset FSHD (73%42), our cohort showed a lower frequency (14% in the whole group, 22% in the early-onset subgroup), which is more in line with classic onset FSHD with de novo mutations in up to 30%43). A correlation between disease severity and the repeat size, as found in earlier research,44 was not demonstrated in this cohort. This could be attributed to the difficulty defining disease severity at a very young age and the cohort size. We did find a shorter repeat length in the early-onset group compared to the classic onset group. In conclusion, the genotype of FSHD in childhood resembles the genotype of FSHD in adults.
This study has identified two important motor features in childhood FSHD. First, we observed a limited functional exercise capacity and structural muscle abnormalities on muscle ultrasonography in patients with normal to mildly reduced muscle strength as measured with manual muscle testing and normal muscle performance on the MFM. This is in line with observations in Duchenne muscular dystrophy showing widespread muscle fibrosis early in the disease course, first resulting in decreased functional exercise capacity followed by muscle weakness45; our data could suggest a similar sequence in FSHD. Another explanation could be the insensitivity of the MFM on muscle performance. Second, we frequently observed postural abnormalities and abdominal muscle involvement on muscle ultrasonography, thereby pointing to early axial muscle involvement in FSHD, as described in adults by others46 and our group.47 In addition, hamstring shortening was frequently observed, which can be caused by increased thoracic kyphosis and which was noted in the sitting position. Early recognition of spinal deformities is important for adequate management of pain and respiratory function.48
The relative low prevalence of central nervous system features in our cohort also supports the heterogeneous clinical spectrum of FSHD in childhood: The frequency of hearing loss, vision loss, epilepsy, and intellectual disability was low and similar to those in adults with classic FSHD.12, 49 The hypothesis that central nervous system signs present later in life is unlikely attributed to the suspected underlying genetic mechanisms.11, 50 The most likely explanation for the discrepancy with previous studies on early-onset FSHD8, 11, 13 is selection and publication bias of the published cases.
Many patients reported fatigue (83%), pain (63%), and a decreased quality of life (70%). This is in accord with a previous study in adults showing high disease burden, with 61% having severe fatigue,51 56% reporting at least mild/moderate pain, and all quality-of-life domains being impaired.52 This decreased quality of life is in contrast to reports on Duchenne muscular dystrophy, where a quality of life similar to healthy peers was found.53 Explanations could be the sampling method of the questionnaires, the burden of facial weakness resulting in impaired social contact, the high frequency of pain,54 and/or a limited functional exercise capacity, which could be amplified by the absence of visible signs of a myopathy.
Based on our results, the management of FSHD in childhood should focus on facial weakness, pain, fatigue, functional exercise capacity, and quality of life. Recommended sensitive outcome measures for clinical trials are the 6-minute walk test, which has been validated and frequently used in other pediatric neuromuscular diseases55, 56 and muscle ultrasonography, which even showed abnormalities in patients with otherwise completely normal neurological examination in our cohort similar to infants with Duchenne.57 In future studies, it would be interesting to look at additional functional exercise capacity tests targeting the upper extremities as well. Currently, there are no evidence-based therapies for FSHD in childhood. It could therefore be useful to test the effect of symptomatic interventions, such as aerobic exercise training58 or cognitive behavioral therapy,59 on fatigue and quality of life in children.
This study has a number of limitations. Most important, missing data were frequent because of the specific patient population. Some patients and parents wished to participate only by home visits or medical file investigations; some patients were too young for testing (minimal ages can be found in Supporting Information Appendix A), or had difficulty with understanding the instruction, or had unsatisfactory motivation (specifically in quality-of-life questionnaires and blood withdrawal; spirometry frequently failed because of inadequate mouth closure). Another limitation is the incomplete inclusion. Four of the 32 patients were not included in the study. However, clinical follow-up showed that 3 were asymptomatic or only mildly affected, and the fourth was lost to follow-up. Therefore, our disease severity may rather be an overestimation than an underestimation. Last, the tests for motor functioning were possibly not sensitive enough; for future testing, we would recommend hand-held dynamometry or quantified muscle testing methods and more exercise capacity measurements.
In this nationwide study, FSHD in childhood is characterized by facial weakness without impaired muscle strength and performance, decreased functional exercise capacity, decreased quality of life, and a broader clinical phenotype and genotype than previously expected. This research will serve as a basis for future natural history studies. Additionally, muscle ultrasonography and the 6-minute walk test are sensitive for disease pathology in childhood FSHD, and therefore additional longitudinal studies on these promising biomarkers are justified.
Acknowledgment
The study is externally funded by a major funding body (charitable foundation Prinses Beatrix Spierfonds / Spieren voor Spieren, W.OR14.22 to C.E. and B.v.E.). There is no commercial party involved.
We thank all patients, parents, and sponsors for their time and effort in this study. In addition, we would like to acknowledge the following people who have contributed to the study: Edith Cuppen, Yvonne Dominicus, Pauline Gans, Bregje Jaeger, Henny Jansen, Nelly Lemmens, Erik Niks, Sytske Nawijn, Joost Nicolai, Maaike Pelsma, Wilma Raijmakers, Vivian Schreur, Bas Stunnenberg, Linda Verhaegh, Astrid Verhoef, and Jack Weeda.
Author Contributions
Authors involved in the study concept and design were: R.G., T.S., N.A., I.G., N.V., S.M., B.E., and C.E. Authors involved in the data acquisition and analysis were: R.G., M.J., T.T., P.V., R.L., S.M., B.E., and C.E. Authors involved in drafting the text and figures were: R.G., T.S., N.A., I.G., N.V., S.M., B.E., and C.E.
Potential Conflicts of Interest
Nothing to report.




