Flunitrazepam overdose induces brilliant-blue gastric fluid
We report the case of a patient with flunitrazepam overdose who presented with brilliant blue-stained gastric fluid.
A 37-year-old woman who had a history of borderline personality disorder was transported to our hospital with disturbance of consciousness by the emergency medical service. On arrival, the Glasgow Coma Scale score was 6/15 (eye 1, verbal 1, motor 4), and both pupils were approximately 3 mm in diameter and reactive to light. Her blood pressure was 104/76 mmHg, heart rate was 64 b.p.m., respiratory rate was 10 breaths/min, and oxygen saturation was 99% on 6 L of oxygen. She did not smell of alcohol, and had airway constriction. Endotracheal intubation was performed, and 100 mL blue-colored gastric fluid was aspirated after nasogastric intubation (Fig. 1). Laboratory results on arrival were as follows: serum sodium level, 141 mmol/L; serum calcium level, 9.0 mg/dL; urea nitrogen level, 6.2 mg/dL; and blood glucose level, 119 mg/dL. Head computed tomography scan yielded normal findings. The emergency medical service informed us about her medical history, and empty press-through packages (32 tablets of 2 mg flunitrazepam, 52 tablets of 5 mg nitrazepam, and 87 tablets of 25 mg quetiapine) were found in her living room. Rapid urine toxicology screening test revealed positive results for benzodiazepines. She was diagnosed with the above drug overdoses, and admitted to our hospital. She was extubated 3 days post-admission, and discharged after a psychiatric examination without any sequelae.
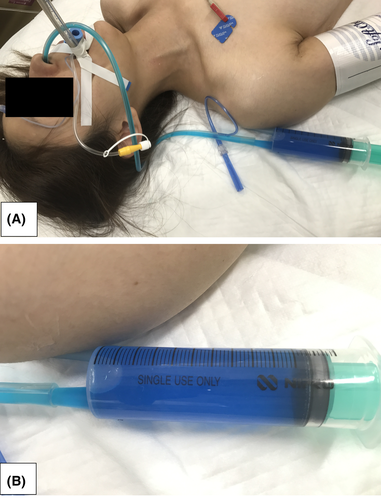
Our case highlighted the brilliant-blue gastric fluid due to flunitrazepam overdose. Flunitrazepam is a central nervous system depressant that belongs to the class of drugs known as benzodiazepines. While flunitrazepam produces sedative-hypnotic, anti-anxiety, and muscle relaxant effects, it is also used in combination with alcohol to produce an exaggerated intoxication, and misused to physically and psychologically incapacitate victims targeted for sexual assault.1 This drug has never been approved for medical use by the US Food and Drug Administration.1 In Japan, the white color of flunitrazepam (Silece; Eisai, Tokyo, Japan, and Rohypnol; Chugai, Tokyo, Japan: Basel, Switzerland) tablets has been changed to blue to prevent its use for criminal purposes by the Ministry of Health since July 1, 2015.2 Although intended to prevent crime, there are some medical merits. If we could not diagnose the cause of the altered consciousness, the blue-colored gastric fluid could signify a possible overdose; we could then anticipate the patient's altered consciousness to resolve, knowing its half-life (10–30 h).3
We retrospectively evaluated the medical records of patients with drug overdose between July 2015 and July 2017 at our institution. Of the 90 patients identified, blue-colored gastric fluid was found in four patients. The diagnosis of flunitrazepam was confirmed with oral evidence in two patients; for one patient it was suspected based on the drug history, but there was no evidence from the medical history of the other patient.
There are several limitations to making a diagnosis based on the blue-colored gastric fluid. First, Silece and Rohypnol expire 4 and 5 years, respectively, from the date of manufacture (both have been changed from 3 years since July 1, 2015). Until June 2020 or later, we may treat patients who present with flunitrazepam overdose due to the old formulation, so flunitrazepam overdose may not always present as blue-colored gastric fluid. Second, flunitrazepam is not the only substance that colors gastric fluid blue. Some drugs such as triazolam (Halcion; Pfizer, New York, New York, United States), digoxin elixir (Digosin elixir; Chugai, Tokyo, Japan), sodium gualenate hydrate (Azunol gurgle liquid; Nitten Pharmaceuticalm, Nagoya, Japan), levodopa carbidopa hydrate (Menesit; Merck & Co., Kenilworth, New Jersey, United States), and aripiprazole (Abilify; Otsuka, Tokyo, Japan), as well as some detergents, are blue substances, and may color gastric fluid blue. However, these drugs and detergents were not suspected as the causative agents in our case. Despite these limitations, emergency physicians should be aware that blue gastric fluid may indicate flunitrazepam overdose.
Disclosure
The institutional review board of Tokyo Metropolitan Bokutoh Hospital approved this case report.Written informed consent was obtained from the patient for publication of this case report and any accompanying pictures. Authors declare no conflict of interest for this article.




