Lung function after matched-related donor allogeneic hematopoietic stem cell transplantation in children with sickle cell disease
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative option for sickle cell disease (SCD) in children. This treatment is reserved for severe forms of SCD that progress despite intensive treatment with transfusion therapy or a hydroxyurea program and require an HLA-compatible family member. Although HSCT has substantially improved survival and reduced risk of complications, it can lead to acute or chronic pulmonary complications, the most common being bronchiolitis obliterans syndrome (BOS) corresponding to a manifestation of chronic graft-versus-host disease (GVHD). After allogeneic HSCT for hematological malignancies, pulmonary complications and impaired pulmonary function are common and result in significant morbidity and mortality.1
Only a few studies have focused on the lung function of SCD patients after allogenic HSCT; they suggest a stability of lung function as compared with patients undergoing HSCT for hematological malignancies. Nevertheless, the small size of the cohorts studied and the lack of data on pre- and posttransplant pulmonary function tests (PFTs) do not allow for an accurate assessment of the pulmonary risks of this treatment in this indication.
In the present study, we retrospectively analyzed the respiratory function of children and adolescents with SCD who underwent geno-identical allogenic HSCT between May 1990 and June 2016 and were prospectively included in the large cohort of the reference center of Creteil, France. Children were eligible if they had at least one lung function assessment after HSCT and were followed clinically for at least 4 years. Evolution of lung function was assessed in a subgroup of patients who had pre- and posttransplant PFTs by comparing the PFT results performed just before allogenic HSCT with the most recent ones during follow-up.
Plethysmography and spirometry were performed and analyzed according to the European Respiratory Society and American Thoracic Society guidelines.2 Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) (FEV1/FVC ratio), and static volumes (vital capacity [VC] and total lung capacity [TLC]) were calculated according to the reference equations proposed by the Global Lung Initiative to account for ethnicity in addition to age, sex and height (http://gligastransfer.org.au/calcs/spiro.html).3 Abnormal lung function was defined as three types of ventilatory defect: obstructive ventilatory defect (OVD) defined by a reduction in FEV1/FVC z-score to less than the lower limit of normal (LLN); restrictive ventilatory defect (RVD) defined by TLC less than LLN confirmed by FVC also below LLN, with normal FEV1/FVC ratio; and mixed ventilatory defect. If no TLC results were available, FVC < LLN with normal FEV1/FVC ratio was considered a restrictive pattern. Severity of RVD was defined in three categories based on z-score: mild (−1.65 to −2.5), moderate (2.51 to −4.0), and severe (≥ −4). The diffusing capacity for carbon monoxide (DLCO), measured with the single-breath technique, was corrected by hemoglobin (DLCOc) and considered pathological when <80%.
All parents provided informed consent for data to be used for research. Use of the database was approved by the Centre Hospitalier Intercommunal de Créteil Institutional Review Board and the French National Data Protection Commission (CNIL, no. 2069568). The study was approved by our institutional ethics committee (no. 2021-06-01).
Between May 1990 and June 2016, 135 patients with SCD underwent geno-identical allograft: 108 had lung function assessments during posttransplantation follow-up and 44 also had pre-allograft PFTs (Figure 1A). The characteristics of the included patients are summarized in Table S1. All patients underwent transplantation at a median age of 8 years (interquartile range 6–12; range 2–20 years). The stem cell source was only umbilical-cord blood or bone marrow. All patients underwent a pretransplantation transfusion program and most (105 of 108) received the same myeloablative conditioning with busulfan and cyclophosphamide chemotherapy combined with rabbit anti-thymocyte globulin, which is routinely used to prevent GVHD and rejection. In the posttransplantation period, the main immunosuppressive treatment was cyclosporine, with or without methotrexate, for a mean (SD) of 10.1 (4.6) months. For 13 patients, cyclosporine was stopped because of neurological or renal toxicity and switched to mycophenolate mofetil. Of the 19 (18%) patients in whom acute (n = 15) or chronic (n = 4) GVHD developed, only one had a pulmonary location, which was associated with a skin location and none had BOS or graft rejection.
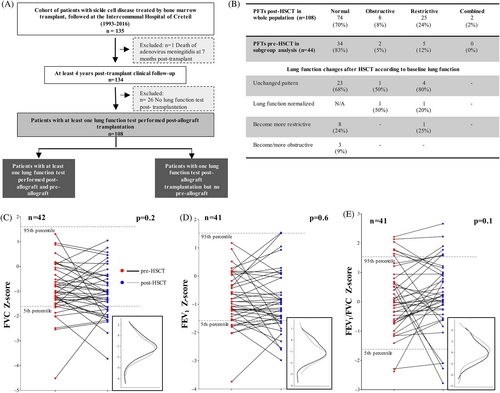
Lung function was assessed in 108 patients after transplantation as was the lung function progression of 44 individuals who had also PFTs before transplantation. Two of the youngest children were unable to perform spirometry and had airway resistance measurement before transplantation. However, their pretransplant airway resistance and lung function were normal after HSCT. The characteristics of this subgroup (n = 44) were comparable to those with only post-PFTs (n = 64), except for age at transplantation and GVHD prevention treatment (Table S1).
In the whole population (n = 108), 74 (70%) patients had normal posttransplant respiratory function at a median of 6 years after transplantation (Figure 1B). The mean (SD) (expressed in z-score for volumes and in percentage for DLCO ± SEM) respiratory function parameters post-allograft were within the normal range (FEV1 = −0.90 [0.78], FEV1/FVC = 0.28 [0.91], TLC = −1.57 [0.68], DLCOc = 96 [14]).
In the subgroup with pretransplant PFTs, the comparison of lung function for each patient at baseline and after allogenic HSCT is shown in Figure 1C–E. The median time between the HCST and PFTs posttransplantation was 5 years (interquartile range 3–7). We found no significant difference in the individual comparison of forced lung volumes before and after allogenic HSCT, as illustrated by superimposing volume distribution curves before and after transplantation. However, we observed a significant and isolated decline in TLC in 26 patients for whom TLC measurement was available before and after HSCT (p = .01). In contrast, 55% of patients showed significantly improved DLCOc (p = .04), with an increase of at least 10% after HSCT.
Before HSCT, 83% (n = 34) of the subgroup of patients with pre- and posttransplant PFTs had normal lung function, 5 (12%) had RVD, and 2 (5%) had OVD without respiratory symptoms (Figure 1B). Overall, 57% of patients with abnormal lung function had a history of acute chest syndrome and one patient with OVD was receiving inhaled corticosteroids for clinically well-controlled allergic asthma. Initially, DLCOc decreased in 11 (25%) patients and was most often isolated (n = 10, 90%). Regardless of lung function at baseline, most patients remained stable after transplantation and two patients showed normalized lung function (Figure 1B). RVD developed in one quarter of patients and OVD in only three patients (9%), without clinical symptoms suggesting BOS. After transplantation, the prevalence of RVD and OVD in the whole cohort was similar to that in the subgroup and was 23% and 7% of the whole population versus 27% and 9% of the subgroup.
To our knowledge, this is the largest cohort with a long follow-up describing the evolution of lung function after transplantation in patients with SCD. Like previous studies, we showed prolonged stability of lung function in most patients who underwent HSCT and very few pulmonary complications, with only one case of acute pulmonary GVHD.4 In our cohort, the most frequent ventilatory defect in the posttransplant period was RVD, with an estimated prevalence of 24%. This prevalence probably varies with age and has been reported from 20% to 30% in similar studies.5 Although we may have missed short-lived abnormalities in the first 1–2 years post-HSCT, we did not find a significant impact of HSCT on lung function and did not observe bronchiolitis obliterans, the dreaded complication after HSCT. However, more than one-quarter of patients exhibited a decrease in lung volumes, particularly TLC and FVC, and restrictive syndrome developed after transplantation, as previously described.5 The type of transplantation and conditioning regimens may play a role in the development of ventilatory defect, but they are mainly associated with a decrease in FVC and FEV1 but not TLC. Furthermore, children with SCD experience a progressive decrease in lung volume with age, starting in childhood and most often resulting in an RVD.6 This situation may explain a decline in lung function in some patients independent of transplantation, which suggests that transplantation does not completely modify the natural course of SCD. However, further studies are needed to evaluate the rate of decline in lung volume per year before and after transplantation to more accurately assess the impact of transplantation on lung function.
As in previous studies, comparison of mean DLCOc before and after allogeneic HSCT showed a significant improvement, with a gain of at least 10%. Although the mechanism remains unclear, the observation may result from an improvement in gas exchange across the alveolar capillary membrane.
Our study has strengths but also limitations: a substantial number of patients did not benefit from pretransplantation PFTs owing to their young age and we did not have a predefined time course for PFT. Although ethnicity was taken into account for spirometry with the Global Lung Initiative equations,3 the ethnic origin indicated was African American, which did not necessarily correspond to SCD patients of African origin living in Europe or patients from the Caribbean.
To summarize, lung function in SCD children undergoing allograft HSCT remained stable after transplantation, without significant pulmonary complications. However, larger prospective studies are needed to confirm these encouraging results in patients with SCD undergoing HSCT.
AUTHOR CONTRIBUTIONS
MG performed the statistical analysis and wrote the original draft of the manuscript. CD conceived and designed the study, wrote and reviewed the manuscript, and verified all data. RE designed the study and wrote and reviewed the manuscript. CP, CA, AK, FB, and BM collected the data, participated in the analysis, and reviewed the manuscript. CD is the study guarantor who attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
ACKNOWLEDGMENTS
We are grateful to Melissa Masson and Naomie Salaün-Penquer (KADUCEO®) for their assistance in the statistical analysis. We are thankful to Laura Smales for English editing assistance.
CONFLICT OF INTEREST STATEMENT
FB reports expert consultancy for BlueBirdBio, Vertex, Global Blood Therapeutics, CP reports honoraria from Novartis, and expert consultancy for Addmedica and Global Blood Therapeutics. The other authors declare no conflicts of interest regarding this paper.
PRINCIPAL INVESTIGATOR STATEMENT
The authors confirm that the PI for this paper is Celine Delestrain and that the PI had direct clinical responsibility for patients.
Open Research
DATA AVAILABILITY STATEMENT
All the data used in this study are available upon request to the corresponding author.




