Rivaroxaban versus nadroparin for thromboprophylaxis following thoracic surgery for lung cancer: A randomized, noninferiority trial
Mengmeng Zhao and Yi Bao contributed equally to this work as co-first authors.
Qiankun Chen, Lei Shen, and Chang Chen contributed equally to this work as co-senior authors.
Abstract
The benefit of rivaroxaban in thromboprophylaxis after oncologic lung surgery remains unknown. To evaluate the efficacy and safety of rivaroxaban, patients who underwent thoracic surgery for lung cancer were enrolled, and randomly assigned to rivaroxaban or nadroparin groups in a 1:1 ratio; anticoagulants were initiated 12–24 h after surgery and continued until discharge. Four hundred participants were required according to a noninferiority margin of 2%, assuming venous thromboembolism (VTE) occurrence rates of 6.0% and 12.6% for patients in the rivaroxaban and nadroparin groups, respectively. The primary efficacy outcome was any VTE during the treatment and 30-day follow-up periods. The safety outcome was any on-treatment bleeding event. Finally, 403 patients were randomized (intention-to-treat [ITT] population), with 381 included in per-protocol (PP) population. The primary efficacy outcomes occurred in 12.5% (25/200) of the rivaroxaban group and 17.7% (36/203) of the nadroparin group (absolute risk reduction, −5.2%; 95% confidence interval [CI], [−12.2–1.7]), indicating the noninferiority of rivaroxaban in ITT population. Sensitivity analysis was performed in the PP population and yielded similar results, confirming the noninferiority of rivaroxaban. In the safety analysis population, the incidence of any on-treatment bleeding events did not differ significantly between the groups (12.2% for rivaroxaban vs. 7.0% for nadroparin; relative risk [RR], 1.9; 95% CI, [0.9–3.7]; p = .08), including major bleeding (9.7% vs. 6.5%; RR, 1.6 [95% CI, 0.9–3.7]; p = .24), and nonmajor bleeding (2.6% vs. 0.5%; RR, 5.2 [95% CI, 0.6–45.2]; p = .13). Rivaroxaban for thromboprophylaxis after oncologic lung surgery was shown to be noninferior to nadroparin.
Abbreviations
-
- ARR
-
- absolute risk reduction
-
- CI
-
- confidence interval
-
- CTPA
-
- computed tomography pulmonary angiography
-
- DOACs
-
- direct oral anticoagulants
-
- DVT
-
- deep vein thrombosis
-
- LMWH
-
- low-molecular-weight heparin
-
- PE
-
- pulmonary embolism
-
- RR
-
- relative risk
-
- VTE
-
- venous thromboembolism
1 INTRODUCTION
The risk of venous thromboembolism (VTE), consisting of pulmonary embolism (PE) and deep vein thrombosis (DVT), after oncologic lung surgery, remains high because of the increased intrapleural pressure ascribed to the artificial pneumothorax; VTE has been reported to be associated with long-term complications, functional disability or death in previous studies.1-5 Additionally, activation of the platelet coagulation cascades, overproduction of procoagulant components due to cancer and surgery, and a prolonged operation time also predispose patients to VTE events following thoracic surgery.6-9 Thus, the American College of Chest Physicians 9th edition guidelines state that there is adequate evidence (grade 1B) to recommend the use of routine in-hospital VTE prophylaxis with unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) in patients who received thoracic surgery.10, 11
However, continuous treatment with LMWH is challenging because of its inconvenience to patients and adverse effects associated with subcutaneous injections, including local pain and bruising,12 contributing to approximately 50% of patients failing to adhere to long-term treatment with parenteral LMWH.13 Recently, rivaroxaban, a direct oral anticoagulant (DOAC) with a rapid onset of action and predictable pharmacokinetics, has been shown to be noninferior or even superior to LMWH in preventing VTE after hip or knee arthroplasty with no significant differences in major bleeding rates.14-23 Most recently, several studies have indicated the potential of DOACs in preventing postoperative VTE after nonmajor orthopedic surgery.24, 25 Thus, oral rivaroxaban is highly accepted by patients due to its efficacy, safety and conveniences, making it an alternative therapeutic option for thromboprophylaxis after orthopedic surgery. However, to the best of our knowledge, no studies have investigated the possibility of rivaroxaban as a potential alternative to LMWH or UFH in thromboprophylaxis for patients with lung cancer after thoracic surgery.
In this context, we designed and conducted this single-blind, noninferiority, randomized clinical trial to investigate whether rivaroxaban is noninferior to nadroparin for postoperative VTE prevention among lung cancer patients who undergo thoracic surgery. We further explored the relative risk of bleeding events and considered whether oral rivaroxaban might be a promising option for thromboprophylaxis in patients who undergo oncologic lung surgery.
2 METHODS
2.1 Ethics statement
This was a single-blind, noninferiority, randomized clinical trial conducted in the Department of Thoracic Surgery of Shanghai Pulmonary Hospital between March 2021 and July 2022, carried out in accordance with the Declaration of Helsinki (as revised in 2013)26 and reported in accordance with the CONSORT guidelines (Appendix S1). The study was approved by the Ethics Committee of our center (L20-221) and registered in the Chinese Clinical Trial Registry (ChiCTR2000038186). Written informed consent was obtained from participants.
2.2 Patient population
Consecutive patients who were hospitalized for pulmonary nodular lesions and were preparing to undergo anatomic lobectomy or segmentectomy from March 1, 2021, to July 15, 2022, were eligible for enrollment in the trial. The inclusion criteria were (1) age ≥18 years; (2) invasive lung cancer confirmed by intraoperative frozen section examination; (3) postoperative Caprini score ≥5; and (4) signed and dated informed consent form. The Caprini score was calculated based on the Caprini model from Boston Medical Center (BMC).27, 28 Patients were excluded if they met any of the following criteria: (1) paraffin section examination-confirmed noninvasive lung cancer; (2) invasive lung cancer history of VTE before surgery; or use of anticoagulant or antiplatelet drugs within 1 week before surgery; (2) active bleeding or high bleeding risk after surgery preventing the receipt of LMWH; (3) contraindications or allergies to anticoagulants; (4) liver disease causing ineffective metabolism of the study drugs; (5) any other contraindication leading to failure of the study drugs; (6) the need for prolonged use of anticoagulants; (7) the need for other preventive anticoagulant therapy in addition to the study drugs; (8) any conditions in which ultrasound or computed tomography pulmonary angiography (CTPA) were contraindicated; (9) pregnancy or lactation; and (10) active infectious disease.
2.3 Random assignment and study interventions
As illustrated in Figure 1, patients were randomly assigned to oral rivaroxaban (Xarelto, Bayer Schering Pharma AG, Germany) or subcutaneous nadroparin (Fraxiparine, Glaxo Smith Kline, France) in a 1:1 ratio. A block randomization scheme was applied in which the block size was only accessible to the statistician (J. L.) who performed the randomization and generated the allocation sequence but otherwise did not participate in the study. Furthermore, opaque, sealed envelopes were distributed to the investigators to conceal the allocation sequence. Once a patient was enrolled in the study, the investigator opened the corresponding random envelope of the patient and allocated the patient into the oral rivaroxaban group or subcutaneous nadroparin group according to the randomization assignment.
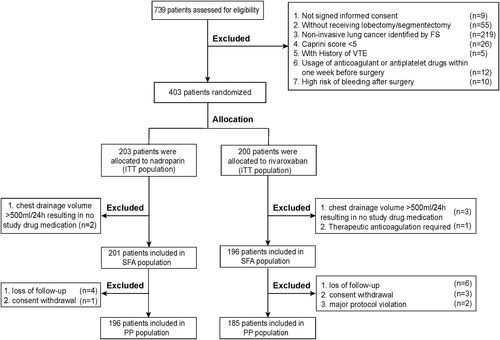
For patients assigned to rivaroxaban, 10 mg was administered orally once daily. For patients assigned to nadroparin, a weight-based dosing, rather than fixed dosing, was employed in our center, since weight-based prophylactic dosing is preferable for patients with extreme body weight.29-32 Under the guidance of the nadroparin drug specifications, 38 units/kg was subcutaneously administered once daily for 3 days postoperatively, and increased to 57 units/kg once daily from fourth postoperative day until discharge. The administration of the study drugs started 12–24 h after surgery and continued once daily every 24 h within a window of ±2 h until discharge from the hospital. The treatment period for each patient was no more than 7 days based on current clinical guidelines,10, 33 and anticoagulant therapy was stopped immediately if suspected bleeding events occurred, such as a chest drainage volume >500 ml in 24 h. All patients included in our trial were followed up 30 days after surgery.
2.4 Outcome measures
All outcomes were assessed by an independent, central adjudication committee whose members were unaware of the study assignments. The primary efficacy outcome was a composite of any DVT or nonfatal PE event within 30 days after surgery. The main secondary efficacy outcome was major VTE, including proximal DVT and nonfatal PE. Other efficacy outcomes included the incidence of DVT (any, proximal, or distal), symptomatic VTE during the treatment period or follow-up period (30 days after surgery), and coagulation abnormalities. DVT was examined by an experienced sonographer (L.S. Xu) with an ultrasound machine (Philips Ultrasound EPIQ5, Philips Healthcare, Bothell, WA, USA) on the day of discharge and postoperative day 30, or earlier if symptoms were present. The ultrasound examination included the deep venous system, including the common femoral vein, deep femoral vein, popliteal, anterior and posterior tibial veins. PE was suspected in patients with sudden shortness of breath, hypoxemia, or cardiac arrest, and confirmed by CTPA.
The safety outcome was the incidence of any bleeding events occurring between intake of the first dose of the study medication and 2 days after the last dose (on-treatment period), including major bleeding and nonmajor bleeding events. Major bleeding after thoracic surgery was defined as bleeding that was fatal, involved a critical organ, required reoperation or clinically overt bleeding outside the surgical site that was associated with a decrease in hemoglobin level ≥20 g/L or required the transfusion of ≥2 units of whole blood or red cells.34 Nonmajor bleeding after thoracic surgery was defined as overt bleeding not meeting the criteria for major bleeding and corresponding to any bleeding necessitating medical intervention or a specific, unscheduled consultation or treatment discontinuation, or resulting in a deterioration of the subject's quality of life.25 The chest drainage volume was recorded daily for 3 days postoperatively. Laboratory variables were also monitored during the treatment and follow-up periods. The criteria for coagulation abnormalities, impaired liver function, impaired renal function and anemia are detailed in Appendix S2.
2.5 Sample size and statistical analysis
The assumption of the VTE occurrence rate for lung cancer patients who received nadroparin after surgery for prophylactic anticoagulation was based on our previous results, in which it was 12.6% (25/198), and the VTE occurrence rate for those patients who received rivaroxaban was based on the Record-217 and Record-3 studies,15 in which it was 6% (2% in the RECORD-2 study and 9.6% in the RECORD-3 study). Thus, a sample of 200 patients for each group was calculated to draw a noninferiority conclusion with a noninferiority margin of 2%, a statistical power of 80%, a two-sided type I error rate of 5%, and a dropout rate of 10%.
Statistical analysis was performed after the data lock. To determine the noninferiority of rivaroxaban to nadroparin, the primary efficacy analysis was performed in the intention-to-treat population (ITT population: all patients who underwent randomization), and further in the per-protocol population (PP population: all patients meeting the inclusion criteria who underwent surgery, received at least one dose of trial medication, and had no major protocol violations) for sensitivity analysis. Noninferiority could be concluded if the upper bound of the 95% CI for the difference in the incidence of VTE between groups was lower than the 2% noninferiority margin. The risk ratio (RR) and its 95% confidence interval (CI) were used to evaluate the logistic regression model, and the absolute risk reduction (ARR) was calculated by using a normal approximation test for patients receiving rivaroxaban versus those receiving nadroparin. Safety analysis was performed in the safety analysis population (SFA population: all patients meeting the inclusion criteria who underwent surgery and received at least one dose of the study trial medication). An additional post hoc analysis was eventually performed to compare the composite of VTE and major bleeding between groups and generate the net clinical benefit derived from rivaroxaban. Statistical analysis was also performed in patients with different times of first study drug administration after surgery, due to potential differences between patients who received anticoagulants in the first and last 12 h.
The analysis of all outcomes was used to compare patients receiving rivaroxaban to the reference group of patients receiving nadroparin. Continuous variables are presented as the mean (standard difference, SD) or median (interquartile range, IQR), and categorical variables are presented as the frequency with percentages, with significant differences compared using SD. This metric describes differences between group means relative to the pooled standard deviation and was considered clinically meaningful if >20%. All statistical analyses were performed by SPSS software (version 23.0, IBM) and R software (version 3.6.2), a two-sided value of p < .05 was considered statistically significant.
3 RESULTS
3.1 Demographic and clinical characteristics
A total of 403 patients in the Shanghai Pulmonary Hospital, including 203 males (50.4%) and 200 females (49.6%) with a mean age of 61.2 (±9.6) years from March 2021 to July 2022, were enrolled and randomly assigned to the rivaroxaban group (n = 200) or nadroparin group (n = 203). The demographic and clinical characteristics of all patients are detailed in Table 1. The most frequent surgery performed was lobectomy (302/403, 74.9%), with a median operation time of 1.8 h (IQR, 1.5–2.0) and blood loss of 51.2 ml (IQR, 20.0–50.0). The median Caprini score was 7.0 (IQR, 7.0–8.0), and the median duration of trial-drug administration was 3.0 days (IQR, 3.0–5.0). Significant standardized differences were not observed in the demographic and clinical characteristics of the patients, notably including the time for the first dose of the study drug, indicating good balance between the two groups after randomization.
| Characteristic | Overall (n = 403) | Rivaroxaban (n = 200) | Nadroparin (n = 203) | SDb |
|---|---|---|---|---|
| Age (mean ± SD, year) | 61.2 ± 9.6 | 61.7 ± 10.1 | 60.7 ± 9.1 | 0.10 |
| Male sex-no. (%) | 203 (50.4) | 107 (53.5) | 96 (47.3) | 0.12 |
| Height (mean ± SD, m) | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.01 |
| Weight (mean ± SD, kg) | 63.9 ± 10.9 | 64.3 ± 9.8 | 63.4 ± 11.8 | 0.09 |
| BMI (mean ± SD, kg/m2) | 23.5 ± 3.2 | 23.8 ± 3.1 | 23.3 ± 3.2 | 0.15 |
| Smoke history-no. (%) | 92 (22.8) | 38 (19.0) | 54 (26.6) | 0.18 |
| Comorbidity-no. (%) | 0.15 | |||
| Hypertension | 95 (23.6) | 44 (22.0) | 51 (25.1) | |
| Diabetes | 32 (7.9) | 12 (6.0) | 20 (9.9) | |
| Coronary heart disease | 1 (0.2) | 0 | 1 (0.5) | |
| Hyperlipidemia | 2 (0.5) | 2 (1.0) | 0 | |
| Malignant tumor history-no. (%) | 20 (5.0) | 11 (5.5) | 9 (4.4) | 0.05 |
| Neoadjuvant therapy-no. (%) | 32 (7.9) | 18 (9.0) | 14 (6.9) | 0.08 |
| Adjuvant therapy-no. (%) | 134 (33.3) | 66 (33.0) | 68 (33.5) | 0.01 |
| Chemotherapy | 128 (31.8) | 65 (32.5) | 63 (31.0) | 0.03 |
| Other therapy | 5 (1.2) | 0 | 5 (2.5) | 0.16 |
| Caprini Score, median (IQR) | 7.0 (7.0–8.0) | 8.0 (7.0–8.0) | 7.0 (7.0–8.0) | 0.06 |
| Organs function | ||||
| ALT, IU/L-no. (%) | 30 (7.4) | 15 (7.5) | 15 (7.4) | 0.04 |
| <10 | 347 (86.1) | 173 (86.5) | 174 (85.7) | |
| 10–49 | 26 (6.5) | 12 (6.0) | 14 (6.9) | |
| ≥49 | ||||
| AST, IU/L-no. (%) | 0.02 | |||
| <34 | 378 (93.8) | 187 (93.5) | 191 (94.1) | |
| ≥34 | 25 (6.2) | 13 (6.5) | 12 (5.9) | |
| BUN, mmol/L-no. (%) | 0.11 | |||
| <3.2 | 9 (2.2) | 3 (1.5) | 6 (3.0) | |
| 3.2–8.2 | 366 (90.8) | 182 (91.0) | 184 (90.6) | |
| ≥8.2 | 28 (6.9) | 15 (7.5) | 13 (6.4) | |
| Cr, μmol/ L-no. (%) | 0.12 | |||
| <53 | 78 (19.4) | 36 (18.0) | 42 (20.7) | |
| 53–97 | 305 (75.7) | 156 (78.0) | 149 (73.4) | |
| ≥97 | 20 (5.0) | 8 (4.0) | 12 (5.9) | |
| Coagulation | ||||
| PT, sec-no. (%) | 0.06 | |||
| 9.4–12.5 sec | 378 (93.8) | 189 (94.5) | 189 (93.1) | |
| ≥ 12.5 sec | 25 (6.2) | 11 (5.5) | 14 (6.9) | |
| TT, sec-no. (%) | 0.19 | |||
| <10.3 | 3 (0.7) | 0 | 3 (1.5) | |
| 10.3–16.6 | 366 (90.8) | 185 (92.5) | 181 (89.2) | |
| ≥16.6 | 34 (8.4) | 15 (7.5) | 19 (9.4) | |
| Platelet/L distribution-no. (%) | 0.14 | |||
| <125 | 8 (2.0) | 7 (3.5) | 1 (0.5) | |
| 125–350 | 383 (95.0) | 189 (94.5) | 194 (95.6) | |
| ≥350 | 12 (3.0) | 4 (2.0) | 8 (3.9) | |
| Operation-no. (%) | 0.02 | |||
| Lobectomy | 302 (74.9) | 149 (74.5) | 153 (75.4) | |
| Segmentectomy | 101 (25.1) | 51 (25.5) | 50 (24.6) | |
| Operation time (h), median (IQR) | 1.8 (1.5–2.0) | 2.0 (1.5–2.0) | 1.8 (1.5–2.0) | 0.11 |
| Estimated blood loss (ml), median (IQR) | 51.2 (20.0–50.0) | 50.0 (20.0–50.0) | 50.0 (20.0–50.0) | 0.11 |
| Histology confirmed by paraffin section-no. (%) | 0.09 | |||
| SCLC | 8 (2.0) | 4 (2.0) | 4 (2.0) | |
| Adenocarcinoma | 323 (80.1) | 159 (79.5) | 164 (80.8) | |
| Squamous cell carcinoma | 46 (11.4) | 22 (11.0) | 24 (11.8) | |
| Others | 26 (6.5) | 15 (7.5) | 11 (5.4) | |
| Pathological stages-no. (%) | 0.03 | |||
| Stage IA | 236 (58.6) | 120 (60.0) | 116 (57.1) | |
| Stage IB | 69 (17.1) | 32 (16.0) | 37 (18.2) | |
| Stage IIA-B | 34 (8.4) | 12 (6.0) | 22 (10.8) | |
| Stage IIIA-C | 61 (15.1) | 34 (17.0) | 27 (13.3) | |
| Stage IVA | 3 (0.7) | 2 (1.0) | 1 (0.5) | |
| Time for the first dose of study drug | ||||
| Median (h, IQR) | 17.7 (14.5–21.0) | 17.7 (14.5–20.9) | 17.6 (14.5–21.2) | 0.01 |
| Distribution-no. (%) | 0.04 | |||
| ≤12 h | 32 (7.9) | 17 (8.5) | 15 (7.4) | |
| >12 h | 371 (92.1) | 183 (91.5) | 188 (92.6) | |
| Anticoagulation duration (d), median (IQR) | 3.0 (3.0–5.0) | 4.0 (3.0–5.0) | 3.8 (3.0–4.0) | 0.06 |
- Abbreviations: ALT, Alanine aminotransferase; APTT, activated partial thromboplastin time; AST, Aspartate transaminase; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; IQR, interquartile range; PT, prothrombin time; SCLC, small cell lung cancer; SD, standardized difference; TT, thrombin time.
- a Percentages may not total 100 because of rounding.
- b Standardized differences provide a measure of the difference between groups divided by the pooled standard deviation; a value >20% (0.2) is interpreted as a meaningful difference between the groups.
3.2 Primary efficacy outcomes
The primary efficacy outcomes of this study trial are described in Table 2. In the ITT population of 403 patients who underwent randomization, primary efficacy outcomes developed in 25 of the 200 (12.5%) patients from the rivaroxaban group and 36 of the 203 (17.7%) patients from the nadroparin group, with an ARR of −5.2% (95% CI: −12.2 to 1.7). The upper limit of the 95% CI of ARR was less than the noninferiority margin of 2%, indicating the noninferiority of rivaroxaban. In terms of secondary efficacy outcomes, major VTE occurred in 2 of the 403 patients (0.5%), 1 (0.5%) in the rivaroxaban group and 1(0.5%) in the nadroparin group, indicating no significant noninferiority. The use of rivaroxaban for thromboprophylaxis after thoracic surgery was associated with a lower risk of DVT (12.5% vs. 17.5%; ARR, −4.7% [95% CI, −11.7 to 2.2]) and a lower risk of symptomatic VTE during the treatment (10.5% vs. 13.3%; ARR, −2.8% [95% CI, −9.1 to 3.5]) and follow-up periods (7.5% vs. 9.9%; ARR, −2.4% [95% CI, −7.8 to 3.1]). Notably, rivaroxaban was not inferior to nadroparin in preventing proximal DVT (ARR, 0.5% [95% CI, −0.5 to 1.5]) or distal DVT only (ARR, −5.2% [95% CI, −12.1 to 1.6]) after oncologic lung surgery. Coagulation abnormalities occurred in 7.5% (15/200) of patients in the rivaroxaban group and 6.4% (13/203) of patients in the nadroparin group, with an ARR of 1.0% (95% CI, −4.0 to 6.0). Similar results were also derived for patients in the subgroup receiving the first dose of study drug in the latter 12 h after surgery (Appendix S2: Table S2).
| Outcomes | Overall (n = 403) | Rivaroxaban (n = 200) | Nadroparin (n = 203) | ARRa (95% CI) | ||
|---|---|---|---|---|---|---|
| No. (%) | 95% CI | No. (%) | 95% CI | |||
| Primary efficacy outcome | 61 (15.1) | 25 (12.5) | 7.9–17.1 | 36 (17.7) | 12.5–23.0 | −5.2 (−12.2–1.7) |
| Secondary efficacy outcomes | ||||||
| Major VTE | 2 (0.5) | 1 (0.5) | −0.5-1.5 | 1 (0.5) | −0.5-1.5 | - |
| Deep-vein thrombosis | 60 (14.9) | 25 (12.5) | 7.9–17.1 | 35 (17.2) | 12.0–22.4 | −4.7 (−11.7–2.2) |
| Proximal DVT | 1 (0.2) | 1 (0.5) | −0.5-1.5 | 0 | - | 0.5 (−0.5–1.5) |
| Distal DVT only | 59 (14.6) | 24 (12.0) | 7.5–16.5 | 35 (17.2) | 12.0–22.4 | −5.2 (−12.1–1.6) |
| Bilateral | 22 (5.5) | 8 (4.0) | 1.3–6.7 | 14 (6.9) | 3.4–10.4 | −2.9 (−7.3–1.5) |
| Unilateral | 37 (9.2) | 16 (8.0) | 4.2–11.8 | 21 (10.3) | 6.2–14.5 | −2.3 (−8.0–3.3) |
| Symptomatic VTE | ||||||
| During treatment | 48 (11.9) | 21 (10.5) | 6.3–14.7 | 27 (13.3) | 8.6–18.0 | −2.8 (−9.1–3.5) |
| During follow-up | 35 (8.7) | 15 (7.5) | 3.8–11.2 | 20 (9.9) | 5.8–14.0 | −2.4 (−7.8–3.1) |
| Coagulation abnormalities | 28 (6.9) | 15 (7.5) | 3.8–11.2 | 13 (6.4) | 3.1–9.9 | 1.0 (−4.0–6.0) |
| Abnormal PT | 22 (5.5) | 13 (6.5) | 3.1–9.9 | 9 (4.4) | 1.6–7.3 | 2.1 (−2.4–6.5) |
| Abnormal TT | 6 (1.5) | 2 (1.0) | −0.4-2.4 | 4 (2.0) | 0.1–3.9 | −1.0 (−3.3–1.4) |
- Note: The primary efficacy outcome was a composite of any deep-vein thrombosis or nonfatal pulmonary embolism. Major VTE was a composite of proximal deep-vein thrombosis or nonfatal pulmonary embolism. Symptomatic VTE included any symptomatic deep-vein thrombosis (proximal or distal) and nonfatal or fatal symptomatic pulmonary embolism in patients of the safety population.
- Abbreviations: ARR, absolute risk reduction; CI, confidence interval; DVT, Deep venous thrombosis; PT, prothrombin time; TT, thrombin time; VTE, venous thromboembolism.
- a The absolute risk reduction, calculated with the use of a normal approximation test, is for patients receiving rivaroxaban, as compared with those receiving nadroparin.
3.3 Sensitivity analysis
As illustrated in Figure 1, a total of 381 patients adhered to the study trial protocol and were included in the sensitivity analysis, including 185 patients from the rivaroxaban group and 196 patients from the nadroparin group; a description of efficacy events was detailed in Table S1. The primary efficacy outcome developed in 15.0% (57/403) of patients in the PP population, with 11.9% (22/185) occurring in the rivaroxaban group and 17.9% (35/196) occurring in the nadroparin group. Thus, the percentage difference for the primary efficacy outcome between the two groups was −6.0% (95% CI, −13.1 to 1.1), confirming the noninferiority of rivaroxaban. The noninferiority of rivaroxaban for thromboprophylaxis following oncological thoracic surgery was further confirmed in the secondary efficacy outcomes, including major VTE rate (0.5% [95% CI, −0.5 to 1.6] vs. 0.5% [95% CI, −0.5 to 1.5]; ARR, 0% [−1.4 to 1.5]) and DVT (11.9% [95% CI, 7.2–16.6] vs. 17.3% [95% CI, 12.0–22.6]; ARR, −5.5% [95% CI, −12.5 to 1.6]).
3.4 Safety analysis
A total of 397 of 403 patients were included in the SFA population, after excluding 6 patients who did not receive a study drug because of a chest drainage volume >500 ml/24 h. On-treatment bleeding events occurred in 12.2% (24/196) of patients from the rivaroxaban group and 7.0% (14/201) of patients from the nadroparin group, showing an RR of 1.9 (95% CI, 0.9–3.7; p = .08; Table 3). Of them, major bleeding occurred in 19 of the 196 (9.7%) patients in the rivaroxaban group, including 13 patients (6.6%) with a decrease in hemoglobin level of ≥20 g/L and 6 patients (3.1%) with a transfusion of ≥2 units of whole blood, whereas major bleeding events were observed in 13 of the 201 (6.5%) patients in the nadroparin group, including 10 patients (5.0%) with a decrease in hemoglobin level of ≥20 g/L and 3 patients (1.5%) requiring a transfusion of ≥2 units of whole blood, with an RR of 1.6 (95% CI, 0.7–3.2; p = .24).
| Outcomes | Overall (n = 397) | Rivaroxaban (n = 196) | Nadroparin (n = 201) | RRa (95% CI) | p* | ||
|---|---|---|---|---|---|---|---|
| No. (%) | 95% CI | No. (%) | 95% CI | ||||
| Any on-treatment bleeding events | 38 (9.6) | 24 (12.2) | 7.7–16.8 | 14 (7.0) | 3.4–10.5 | 1.9 (0.9–3.7) | 0.08 |
| Major bleeding | 32 (8.1) | 19 (9.7) | 5.6–13.8 | 13 (6.5) | 3.1–9.9 | 1.6 (0.7–3.2) | 0.24 |
| Causing a fall in hemoglobin level of ≥20 g/L | 23 (5.8) | 13 (6.6) | 3.1–10.1 | 10 (5.0) | 2.0–8.0 | 1.4 (0.6–3.2) | 0.48 |
| Causing a transfusion of ≥2 units of whole blood or red cells | 9 (2.3) | 6 (3.1) | 0.6–5.5 | 3 (1.5) | −0.2-3.2 | 2.1 (0.5–8.5) | 0.30 |
| Non-major bleeding | 6 (1.5) | 5 (2.6) | 0.3–4.8 | 1 (0.5) | −0.5-1.5 | 5.2 (0.6–45.2) | 0.13 |
| Surgical site bleeding causing discontinuation of the study drug | 5 (1.3) | 4 (2.0) | 0.1–4.0 | 1 (0.5) | −0.5-1.5 | 4.2 (0.5–37.6) | 0.20 |
| Leading to transfusion of <2 units of blood | 1 (0.3) | 1 (0.5) | - | - | - | 0.99 | |
| Chest drainage volume (median [IQR], ml) | |||||||
| Day 1 | 150.0 (130.0–300.0) | 150.0 (121.25–300.0) | 200.0 (130.0–300.0) | - | 0.52 | ||
| Day 2 | 130.0 (100.0–200.0) | 130.0 (100.0–200.0) | 130.0 (100.0–200.0) | - | 0.64 | ||
| Day 3 | 100.0 (30.0–150.0) | 100.0 (50.0–160.0) | 100.0 (21.25–150.0) | - | 0.26 | ||
| Organ function | |||||||
| Possibility of anemia | 16 (4.0) | 9 (4.6) | 1.7–7.5 | 7 (3.5) | 0.9–6.0 | 1.3 (0.5–3.6) | 0.58 |
| Impaired liver function | 41 (10.3) | 23 (11.7) | 7.2–16.2 | 18 (9.0) | 5.0–12.9 | 1.4 (0.71–2.6) | 0.36 |
| Impaired renal function | 29 (7.3) | 15 (7.7) | 3.9–11.4 | 14 (7.0) | 3.4–10.5 | 1.1 (0.5–2.4) | 0.79 |
| Net clinical benefitb | 93 (23.4) | 45 (23.0) | 17.1–28.8 | 48 (23.9) | 18.0–29.8 | 0.9 (0.6–1.5) | - |
- Note: On-treatment bleeding events included those beginning after the initiation of the study drug and up to 2 days after the last dose of the study drug.
- Abbreviations: CI, confidence interval; IQR, interquartile range.
- a The relative risk, calculated with the use of logistic-regression model, is for patients receiving rivaroxaban, as compared with those receiving nadroparin.
- b Net clinical benefit was assessed in a post hoc analysis that compared the composite of VTE or major bleeding between groups. Because this was a post hoc analysis, no statistical test was performed.
- * The listed p values were calculated with the use of Fisher's exact test for category variables, and Mann–Whitney U test for continuous variables.
Additionally, 6 (1.5%) patients experienced nonmajor bleeding events, including 5 patients (2.6%) from the rivaroxaban group and 1 patient (0.5%) from the nadroparin group (RR, 5.2 [95% CI, 0.6–45.2]; p = .13), showing no significant difference (Table 3). During the first three postoperative days, the cumulative volume of chest drainage decreased every day in both groups, and there was no significant difference in the chest drainage volume between the two groups (p = .52 for the first day; p = .64 for the second day and p = .26 for the third day; Table 3). The occurrence of anemia, impaired liver function, and impaired renal function was also recorded, and did not show significant difference between the two groups (p = .58 for anemia; p = .36 for impaired liver function and p = .79 for impaired renal function; Table 3).
In post hoc analysis, the percentage of patients with a net clinical benefit outcome was lower in the rivaroxaban group (23.0%, 45/196) than in the nadroparin group (23.9%, 48/201), corresponding to a higher incidence in the nadroparin group (RR, 0.9 [95% CI, 0.6–1.5]; Table 3). Similar results for safety outcomes were also derived for patients in the subgroup receiving the first dose of study drug in the latter 12 h after surgery (Appendix S2: Table S3).
3.5 Discussion
In this trial of thromboprophylaxis after oncologic lung surgery, we found that rivaroxaban was noninferior to nadroparin in preventing VTE, with no significant difference in bleeding events. Rivaroxaban reduced the ARR of the composite of any DVT and nonfatal PE event by 5.2% (95% CI, −12.2 to 1.7). This is the first study to compare the efficacy and safety of DOACs with LMWH in postoperative thromboprophylaxis for patients with lung cancer and receiving thoracic surgery, showing that oral, once-daily rivaroxaban has potential for thromboprophylaxis following oncologic lung surgery.
A significant proportion of thoracic procedures are performed for oncology patients, especially for those with lung cancer, since surgical resection is the first-line treatment in many therapeutic strategies. VTE, composed of PE and DVT, is a well-documented cause of morbidity and mortality in the subset of oncologic surgery patients who undergo thoracic surgery,2, 35-37 and patients with lung cancer have been shown to be associated with a greater risk of VTE than those with other solid organ malignant tumors.2, 6, 38, 39 The risk factors for VTE in patients with lung cancer are most likely a combination of patient-related factors, such as age, obesity, and smoking, as well as cancer-related factors, including biology, histologic features, and disease stages.40-42 Furthermore, patients with lung cancer are more likely to experience postoperative VTE due to higher levels of discomfort associated with chest incisions, reduced postoperative ambulation, and extended periods of bed rest. Additionally, other risk factors related to treatment, such as whether the patient had a thoracotomy or the amount of tissue resected, are highly associated with a higher risk for VTE.41, 43 There is little similarity in the reported incidences of VTE after thoracic surgery, with rates ranging from 1.3% to 15.2% for PE and 4% to 14% for DVT.6, 38, 44 Moreover, a recent study showed that 2.1% of patients who had a lobectomy bled afterward, and 1.4% of those patients had to undergo a second surgery.45 Thus, patients hospitalized for active cancer, especially those undergoing major surgery, are categorized as a population with a high risk for VTE according to the Caprini risk score by most guidelines, and thus, pharmacological thromboprophylaxis is of great necessity.10, 27, 28
Pharmacological prophylaxis with UFH or LMWH is recommended to be initiated preoperatively (2–12 h prior to surgery) and given postoperatively within 6–12 h, as per the current guidelines.46 In recent years, it has been clinically shown that LMWH may be as effective as low-dose subcutaneous UFH for thromboprophylaxis in patients undergoing orthopedic or general surgery; however, the risk of bleeding complications is lower with LMWH.47-49 Furthermore, long-term therapy using LMWH has been reported to be linked to fewer heparin-related complications, such as osteoporosis, allergic skin reactions, and heparin-induced thrombocytopenia, than UFH.50 The higher bioavailability, longer half-life and resulting predictable anticoagulant effect of LMWH also allow for its use in the outpatient setting. In particular, LMWH can be given as a once-daily subcutaneous injection, which would be more comfortable for patients, save nursing time, and allow home treatment for patients who require extended prophylaxis. For these reasons, LMWH has now replaced UFH as the preferred prophylactic treatment in general surgery.51, 52 Despite this evidence, the use of subcutaneous LMWH has not been shown to be satisfactory for a number of reasons. Common complaints related to LMWH use include injection site reactions, auto-injection devices, bruising, bleeding, cost, and potential vomiting and nausea in patients with early-stage lung cancer who did not receive chemotherapy, leading to less than 50% of patients adhering to long-term treatment with parenteral LMWH.13
Some DOACs, including rivaroxaban, apixaban, and edoxaban, have been recently shown to be at least as effective as LWMH in the primary prevention of VTE in orthopedic surgeries and in ambulatory high-risk cancer patients or high-risk medical patients, thus making it a potentially safe alternative strategy to LMWH for reducing the risk of a thrombotic event.53 The clinical potential of DOACs has also been demonstrated in patients who have undergone nonmajor orthopedic surgeries of the lower limbs,25 minimally invasive esophagectomy for esophageal cancer,54 and in female patients undergoing surgery for gynecologic malignant neoplasms.24 In patients who have undergone anatomic lobectomy or segmentectomy for lung cancer, our current study found that the usage of rivaroxaban in postoperative prophylactic anticoagulation could reduce the risk of any VTE events by 5.2% (95% CI, −12.2 to 1.7), and of distal DVT events by 5.2% (95% CI, −12.1 to 1.6), indicating the noninferiority of the primary efficacy results derived from rivaroxaban. Our study is the first to evaluate the potential value of DOACs in preventing VTE in patients who undergo thoracic surgery for lung cancer based on a relatively large sample size. In terms of safety outcomes, rivaroxaban and nadroparin were found to have similar safety profiles in our study. There were no clinically significant differences in the incidence of bleeding or other safety outcomes between the two groups. However, patients in the rivaroxaban group tended to have more bleeding (12.2%, 24/196) than those in the nadroparin group (7.0%, 14/204), with an RR of 1.9 (95% CI, 0.9–3.7; p = .08), in accordance with previously published studies.55-57 Rivaroxaban metabolism occurs in the liver and kidney; therefore, caution should be taken for patients with organ function changes. This study found that there was no significant difference in organ function after study drug administration, or chest drainage volume during the first three postoperative days. According to the above results, our study suggests that rivaroxaban could act as an effective option for postoperative prophylactic anticoagulation and did not increase the risk of postoperative bleeding. Based on our findings, the application scenario for DOACs could be expanded, and more lung cancer patients who undergo thoracic surgery could benefit from DOACs, showing a lower rate of VTE events, thus reducing VTE-related morbidity and mortality.9 Further, lung cancer, along with central nervous system, pancreatic, upper gastrointestinal cancer, had a significantly higher risk of PE than other malignant cancers,39 even though the overall risk of VTE event for lung cancer was intermediate when compared with other cancers.58 Timely anticoagulation prophylaxis is an important method to reduce the incidence of VTE event in cancer patients, especially in lung cancer patients. Our study brought more data to the existing evidence in VTE prophylaxis management for lung cancer population.
Our study had some limitations. Firstly, our institution is a large, referral clinical center in China, and generally treats high-risk patients with anatomically complex tumors, for whom the surgical technologies performed are difficult, which might explain the relatively high incidence of postoperative bleeding. Secondly, the study was conducted with a limited number of participants at a single center. Thirdly, our study was focused on the comparison between rivaroxaban and nadroparin, and all the results concluded from this study might not be directly extrapolated to other LMWHs (e.g., enoxaparin, dalteparin) or DOACs (e.g., edoxaban, apixaban) because of the inalterability during LMWH or DOACs administration.59-65 Finally, only patients who underwent anatomical segmentectomy or lobectomy for lung cancer were enrolled in our study. A prospective, multicenter clinical trial with a larger sample of patients who undergoing other kinds of surgical procedures for lung cancer, such as wedge resection, pneumonectomy, and sleeve lobectomy, is warranted to confirm our findings.
In conclusion, VTE prophylaxis with 10 mg rivaroxaban once daily had a noninferior efficacy outcome to recommended weight-based nadroparin among patients hospitalized for lobectomy or segmentectomy for lung cancer, with no increase in the risk of bleeding events. Therefore, rivaroxaban could be a potential replacement for thromboprophylaxis after oncologic lung surgery.
AUTHOR CONTRIBUTION
Mengmeng Zhao, Yi Bao, Linsong Chen, and Chang Chen carried out the concepts, design, literature search, data acquisition, data analysis, and manuscript preparation. Chao Jiang, Linsong Chen, Lisha Xu, Xiaogang Liu, Jiaqi Li, Yang Yang, Gening Jiang, Jian Li, and Yunlang She aided with data acquisition, data analysis, and statistical analysis. Qiankun Chen, Lei Shen, and Chang Chen carried out the definition of intellectual content, critical revision, and supervision of this article. All authors have read and approved the content of the manuscript. The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
The authors are grateful to the clinical research coordinator, Zhiping Dai, who helped to enroll participants. Then, the authors thank all of the participants, without whom this research would not have been possible.
FUNDING INFORMATION
The study was supported by the Clinical Research Foundation of Shanghai Pulmonary Hospital (FKLY20016), the National Natural Science Foundation of China (91959126, 82102126), the Science and Technology Commission of Shanghai Municipality (20XD1403000, 21Y11913400, 21YF1438200, 201940192).
CONFLICT OF INTEREST STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




