Clinical characteristics and overall survival among acute myeloid leukemia patients with TP53 gene mutation or chromosome 17p deletion
Abstract
Approximately 5% to 15% of acute myeloid leukemia (AML) patients have TP53 gene mutations (TP53m), which are associated with very poor outcomes. Adults (≥18 years) with a new AML diagnosis were included from a nationwide, de-identified, real-world database. Patients receiving first-line therapy were divided into three cohorts: venetoclax (VEN) + hypomethylating agents (HMAs; Cohort A), intensive chemotherapy (Cohort B), or HMA without VEN (Cohort C). A total of 370 newly diagnosed AML patients with TP53m (n = 124), chromosome 17p deletion (n = 166), or both (n = 80) were included. The median age was 72 years (range, 24–84); most were male (59%) and White (69%). Baseline bone marrow (BM) blasts were ≤30%, 31%–50%, and >50% in 41%, 24%, and 29% of patients in Cohorts A, B, and C, respectively. BM remission (<5% blasts) with first-line therapy was reported in 54% of patients (115/215) overall, and 67% (38/57), 62% (68/110), and 19% (9/48) for respective cohorts (median BM remission duration: 6.3, 6.9, and 5.4 months). Median overall survival (95% CI) was 7.4 months (6.0–8.8) for Cohort A, 9.4 months (7.2–10.4) for Cohort B, and 5.9 months (4.3–7.5) for Cohort C. There were no differences in survival by treatment type after adjusting for the effects of relevant covariates (Cohort A vs. C adjusted hazard ratio [aHR] = 0.9; 95% CI, 0.7–1.3; Cohort A vs. B aHR = 1.0; 95% CI, 0.7–1.5; and Cohort C vs. B aHR = 1.1; 95% CI, 0.8–1.6). Patients with TP53m AML have dismal outcomes with current therapies, demonstrating the high unmet need for improved treatments.
1 INTRODUCTION
Acute myeloid leukemia (AML) is among the most common types of leukemia in adults, generally affecting individuals ≥65 years of age.1 Approximately 5% to 15% of patients with AML are known to have TP53 gene mutations (TP53m).2-5 TP53m are also common in patients with therapy-related (30%) and secondary AML (18%).6, 7 TP53 is a tumor suppressor gene that encodes p53, a transcription factor that is activated in response to various forms of cellular stress, triggering multiple antiproliferative and apoptosis functions and, as recently demonstrated, influencing immune regulation; it is also among the most frequently mutated genes in human cancer.6, 8 While sequencing-based analysis of TP53m status is widely available, until recently, patients with AML were not routinely screened specifically for TP53m.9 In addition to loss-of-function mutations in the TP53 gene, deletions of chromosome 17p (del17p) commonly involve the TP53 gene on the short arm of chromosome 17 and result in an allelic loss of the gene with a functionally similar impact as loss-of-function TP53m.10 Most TP53m result in impaired function of the p53 protein, conferring malignant cells an innate resistance to chemotherapy and prolonged survival.2 Patients with TP53m AML have lower overall survival (OS) due to resistance to standard AML therapies and an increased likelihood of relapse after chemotherapy with or without allogeneic hematopoietic stem cell transplantation (allo-HSCT) when compared with patients who have wild-type TP53 (TP53wt).2, 5, 11, 12
TP53m is frequently associated with adverse cytogenetics, such as complex karyotypes, monosomal karyotypes, and specific chromosomal aneuploidies.3, 6, 7, 11 One study found that AML with complex karyotypes accounts for 10% to 15% of cases among adults, with approximately 60% of patients also harboring a TP53m.11 Complex karyotype and TP53m independently confer adverse risk, with the presence of both TP53m and complex karyotype portending a worse clinical outcome than complex karyotype with an unaltered TP53 (median OS [mOS], 4 vs. 11 months).11, 13
First-line (1L) treatment options for AML include intensive chemotherapy (IC), hypomethylating agents (HMAs) with or without venetoclax (VEN), and allo-HSCT, depending on patients' age, fitness, organ function, and molecular/cytogenetic status. The standard of care (SOC) for patients with AML and TP53wt, provided they are fit for intensive treatment, is anthracycline and cytarabine-based induction chemotherapy; however, HMAs have emerged as the SOC in TP53m AML.2 VEN in combination with HMAs (azacitidine [AZA] or decitabine [DEC]) is the preferred 1L treatment for AML patients who are considered unsuitable for induction therapy due to comorbidities or age >75 years.2, 14
Favorable clinical responses, including complete remission (CR) + CR with incomplete blood count recovery (CRi) with an overall response rate of 46%, were seen in a single-institution trial of patients with TP53m (n = 21), TP53wt (n = 78), and TP53 not evaluated (n = 17) treated with a 10-day DEC regimen.15 However, CR + CRi responses were not replicated in a randomized Phase 2 study comparing 5-day (n = 28) versus 10-day (n = 43) DEC treatment regimens.16 Among patients treated with IC or low-intensity chemotherapy, those with TP53m have shown worse remission rates and OS when compared with patients with TP53wt.3 Patients with TP53m AML treated with VEN + HMA experienced inferior outcomes relative to the overall AML patient population in a Phase 1b study (mOS of 7.2 months in TP53m AML patients vs. 17.5 months in all patients).14 In a post hoc analysis of a separate single-arm, single-institution Phase 2 study, treatment of AML with VEN + 10-day DEC resulted in inferior outcomes, including lower remission rates of CR + CRi (57% vs. 77%), lower OS (5.2 vs. 19.4 months), and a higher 60-day mortality rate (26% vs. 4%) in patients with TP53m (n = 35) versus patients with TP53wt (n = 83).17 In a pooled analysis that included patients from the randomized Phase 3 VIALE-A study and a prior nonrandomized Phase 1b study of HMA with VEN, poor-risk cytogenetic patients with TP53m treated with VEN + AZA (n = 54) versus AZA alone (n = 18) had CR + CRi rates of 41% versus 17% and mOS of 5.2 versus 4.9 months, whereas poor-risk cytogenetic patients with TP53wt treated with VEN + AZA (n = 50) versus AZA alone (n = 22) had CR + CRi rates of 70% versus 23% and mOS of 23.4 versus 11.3 months, highlighting the significant prognostic impact of TP53m even in the context of poor-risk cytogenetics.18
There are no established or approved therapies specifically targeting TP53m in AML; therefore, evaluating outcomes among a large cohort of AML patients with TP53m in real-world settings treated with current 1L SOC regimens may help provide important benchmarks for new drug development. The objectives of our study were (1) to evaluate the effectiveness of different SOC (IC, HMA, and VEN + HMA) in treatment-naïve patients with TP53m AML, and (2) to assess the clinical benefits of VEN + HMA therapy compared with IC or HMA alone for adult patients with TP53m AML using data from a nationwide, de-identified, real-world database.
2 METHODS
The study analyzed data from the nationwide electronic health record (EHR)-derived longitudinal Flatiron Health database, comprised of de-identified, patient-level, structured, and unstructured data curated via technology-enabled abstraction originating from ~280 US cancer clinics (~800 sites of care).19, 20 Most patients in the database come from community oncology settings.20
Adult (≥18 years) patients who received new AML diagnoses from January 1, 2014 to February 28, 2021 and relevant structured and unstructured data were identified via International Classification of Disease (ICD) version 9 and 10 codes (ICD-9: 205.0x, 206.0x, 207.0x, 207.2x; ICD-10: C92.0x, C92.5x, C92.6x, C92.Ax, C93.0x, C94.0x, C94.2x).21, 22 To ensure the minimum necessary clinical information was available, patients were required to have at least two clinic encounters on different days occurring on or after January 1, 2014, and to have received 1L therapy within 60 days of diagnosis. Patients from the following groups were excluded from data analysis: (a) patients who received allo-HSCT within 6 months prior to the start of 1L therapy; (b) patients who received any documented investigational drugs or prior antileukemic therapy (except hydroxyurea, oral etoposide, or HMAs for myelodysplastic syndrome [MDS]); (c) patients who had other concurrent malignancies (except treated basal cell carcinoma, localized squamous skin carcinoma, or localized prostate cancer) for which they were receiving anticancer therapy at the time of AML diagnosis; or (d) patients who had acute promyelocytic leukemia (ICD-10: C92.4x), unspecified myeloid leukemia (ICD-9: 205.9x; ICD-10: C92.9x), or acute panmyelosis with myelofibrosis (ICD-10: C94.4x).21, 22 All patients had TP53m status determined from molecular tests or del17p identified by cytogenetic/fluorescent in situ hybridization within 30 days prior to the AML diagnosis date. Only those patients with documented TP53m or del17p were included (Figure 1).
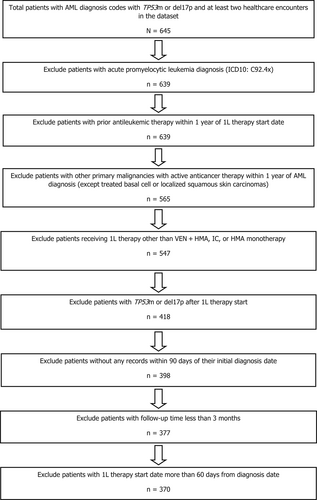
Patients receiving 1L therapy were divided into three cohorts: VEN + HMA (AZA/DEC; Cohort A), IC (Cohort B), or HMA without VEN (Cohort C). Cohort B included cytarabine + daunorubicin/idarubicin, cladribine-based regimens (e.g., CLAG, CLAG-Ida, CLAG-M, CLIA), fludarabine-based regimens (e.g., FLAG, FLAG-Ida, FLAG-M), and MEC (mitoxantrone + etoposide + Ara-C). The study population was restricted to a start date for 1L therapy at least 3 months prior to the study data cutoff date (February 28, 2021) to allow for a minimum of 3 months of follow-up for all patients. The index date was defined as the start date of 1L therapy.
In addition to patients' baseline demographic (age, sex, race, comorbidities, and AML diagnosis year) and clinical characteristics (Eastern Cooperative Oncology Group [ECOG] score; secondary AML; therapy-related AML; World Health Organization classifications; cytogenetic risk categories; bone marrow [BM] blast percentage at diagnosis; chromosome 5, 7, or 17 deletion), data on clinical outcomes (allo-HSCT, BM blast clearance) and death were collected. EHR data were curated for death information, while patient-level mortality data from the Social Security Death Index and a commercial data source that mines obituary and funeral home data were included through deterministic linkage.23 A composite mortality variable was developed by Flatiron Health using structured and unstructured EHR-derived data linked to a commercial death data source and the US Social Security Death Index. Mortality data were extracted from the EHR structured data field “patient date of death,” if available; otherwise, the patient's last activity date was used.
Age was categorized into <65 years, 65 to 74 years, and ≥75 years. Patients with TP53m, del17p, or both, were reported. Patient records were checked for a list of comorbidities included in the Charlson Comorbidity Index (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disorder, peptic ulcer disease, mild chronic liver disease, moderate or severe chronic liver disease, diabetes with end organ damage, diabetes without end organ damage, hemiplegia, moderate to severe renal disease, and HIV/AIDS) prior to 1L therapy.24 Comorbidities were abstracted only from physicians' documentation and not derived from lab values or ICD codes in structured data. BM blast percentages at baseline were divided into ≤30%, 31% to 50%, and >50%. Cytogenetic risk categories based on European LeukemiaNet 2017 criteria (favorable/low risk, intermediate risk, poor/adverse/high risk) were also reported by TP53m status for each cohort, as available.13
Missing/unknown values >2% were reported where applicable. OS was defined as time from 1L therapy start to death. As only month and year were reported, the 15th of the month was imputed as the date of death. Patients without mortality data were censored at the last confirmed activity date. The mOS was estimated using the Kaplan–Meier method. As baseline clinical characteristics could impact both treatment choice and survival, multivariate Cox proportional hazards models (including age, time to 1L therapy, comorbidities, TP53m status, cytogenetic risk level,13 presence of del17p, secondary and therapy-related AML, healthcare practice type, ECOG score, BM blast percentage, and post-1L allo-HSCT status) were used to evaluate the influence of treatment on OS. All analyses were conducted using SAS software (version 9.4, Cary, NC, USA).
3 RESULTS
A total of 370 patients with newly diagnosed AML and either TP53m (n = 124) or del17p (n = 166), or both (n = 80), were included in the study. Baseline demographics and clinical characteristics are summarized in Table 1. Median age was 72 years (range, 24–84), and the majority of patients were male (59%) and White (69%). Treatment Cohorts A, B, and C included 94 (25%), 135 (36%), and 141 (38%) unique AML patients, respectively. Patients in Cohorts A and C were older (median 75 and 77 years, respectively, vs. 62 years in Cohort B) and had more comorbidities (65% and 60% with at least one comorbidity, respectively vs. 40% in Cohort B).
| Overall | VEN + HMA | IC | HMA | |
| N = 370 | n = 94 | n = 135 | n = 141 | |
| Age, years, median (Q1, Q3) | 72 (63, 78) | 75 (69, 80) | 62 (56, 68) | 77 (72, 80) |
| Male | 219 (59) | 56 (60) | 75 (56) | 88 (62) |
| Comorbidities | ||||
| None/1/≥2 | 171 (46)/101 (27)/98 (27) | 33 (35)/25 (27)/36 (38) | 81 (60)/34 (25)/20 (15) | 57 (40)/42 (30)/42 (30) |
| Follow-up time, median (Q1, Q3) | 6 (3, 11) | 6 (3, 9) | 7 (4, 13) | 5 (2, 11) |
| Cytogenetic risk category (ELN 2017) | ||||
| Favorable-low risk | 1 (0.3) | 1 (1) | 0 | 0 |
| Intermediate risk | 5 (1) | 0 | 4 (3) | 1 (1) |
| Poor-adverse-high risk | 240 (65) | 58 (62) | 91 (67) | 91 (65) |
| Missing | 124 (34) | 35 (37) | 40 (30) | 49 (35) |
| TP53ma | 204 (55) | 73 (78) | 77 (57) | 54 (38) |
| With chromosome 17p deletion, n/n (%)b | 80/168 (48) | 30/61 (49) | 28/58 (48) | 22/49 (45) |
| Without chromosome 17p deletion, n/n (%)b | 88/168 (52) | 31/61 (51) | 30/58 (52) | 27/49 (55) |
| Chromosome 17p deletionc | 246 (67) | 51 (54) | 86 (64) | 109 (77) |
| With TP53m, n/n (%)b | 80/97 (82) | 30/34 (88) | 28/34 (82) | 22/29 (76) |
| Without TP53m, n/n (%)b | 17/97 (18) | 4/34 (12) | 6/34 (18) | 7/29 (24) |
| Chromosome 5, 7, or 17 deletion | 324 (88) | 80 (85) | 115 (85) | 129 (92) |
| >1 deletion (chromosome 5, 7, 17) | 251 (68) | 64 (68) | 86 (64) | 101 (72) |
| Therapy-related AML | ||||
| Yes/no or unknown | 38 (10)/332 (90) | 15 (16)/79 (84) | 11 (8)/124 (92) | 12 (9)/129 (92) |
| Secondary AML | 118 (32) | 32 (34) | 37 (27) | 49 (35) |
| Prior treatment with HMA,d n (%) | 26 (7) | 10 (11) | 7 (5) | 9 (6) |
| Allo-HSCT treatment | 30 (8) | 5 (5) | 21 (16) | 4 (3) |
| ECOG at baseline | ||||
| 0–1/≥2/missing | 163 (44)/57 (15)/150 (41) | 48 (51)/21 (22)/25 (27) | 43 (32)/9 (7)/83 (62) | 72 (51)/27 (19)/42 (30) |
| BM blasts at baseline | ||||
| ≤30%/31%–50%/>50% | 151 (41)/88 (24)/107 (29) | 40 (43)/21 (22)/28 (30) | 45 (33)/34 (25)/45 (33) | 66 (47)/33 (23)/34 (24) |
| Time from Dx to 1L Tx in months, median (range) | 0.3 (0.0, 1.8) | 0.4 (0.0, 1.6) | 0.2 (0.0, 1.8) | 0.4 (0.0, 1.7) |
| Allo-HSCT treatment during follow-up | 30 (8) | 5 (5) | 21 (16) | 4 (3) |
| Time to allo-HSCT in months, median (range) | 4.0 (2.0, 10.3) | 5.4 (2.8, 5.8) | 3.7 (2.0, 10.3) | 5.4 (4.4, 9.0) |
| BM blast clearance ratee | 115/215 (54) | 38/57 (67) | 68/110 (62) | 9/48 (19) |
| Time to BM blast clearance rate in months, mean (SD) | 1.9 (1.9) | 2.4 (1.6) | 1.1 (0.8) | 4.9 (3.9) |
| Duration of blast clearance in months, median (95% CI)f | 6.7 (5.6, 7.9) | 6.3 (4.3, 8.3) | 6.9 (5.7, 9.0) | 5.4 (1.7, 14.1) |
| Deceased | 294 (80) | 66 (70) | 101 (75) | 127 (90) |
- Note: Data are presented as n (%) unless stated otherwise. Comorbidities include myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disorder, peptic ulcer disease, mild chronic liver disease, moderate or severe chronic liver disease, diabetes with end organ damage, diabetes without end organ damage, hemiplegia, moderate to severe renal disease, and HIV/AIDS.
- Abbreviations: 1L, first-line; Allo-HSCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; AZA, azacitidine; BM, bone marrow; CMML/CMMoL, chronic myelomonocytic leukemia; DEC, decitabine; Dx, diagnosis; ECOG, Eastern Cooperative Oncology Group; ELN, European LeukemiaNet; HMA, hypomethylating agents; IC, intensive chemotherapy; MDS, myelodysplastic syndromes; TP53m, tumor protein P53 gene mutation; Tx, treatment; VEN, venetoclax.
- a Chromosome 17p deletion status was unknown in 36 patients.
- b Percentages calculated using data from patients with known mutation status.
- c TP53m status was unknown in 149 patients.
- d Twenty-five patients received AZA or DEC as treatment for prior MDS, and one patient received such treatment for CMML/CMMoL.
- e BM blast clearance rate is defined as the percentage of patients who had at least one record of BM blast ≤5% after the start of 1L AML therapy and prior to initiation of next-line therapy or stem cell transplant. The denominator of BM blast clearance rate only includes patients in the full analysis set who had both baseline BM blast >5% and at least one evaluable postbaseline BM blast record.
- f Duration of blast clearance (months) = (earlier date of the first blast >5% post-blast clearance or death—date of BM blast clearance + 1)/30.4375.
Approximately 70% of patients were diagnosed in 2017 or later, and all patients receiving VEN were included from those years, as VEN was approved in 2018 (data not shown). The median follow-up time for the overall cohort was 6 months (range, 0–74) and was comparable among Cohorts A, B, and C (6 [0–20], 7 [0–74], and 5 [0–47] months, respectively; Table 1).
Of patients with TP53m with known del17p status (n = 168), 48% also had del17p, while 52% did not; of patients with del17p with known TP53m status (n = 97), 82% also had TP53m, while 18% did not. Deletions in chromosomes 5, 7, or 17 were reported in 88% (n = 324) of all patients, with 68% having more than one deletion. Secondary AML was reported in 118 (32%) patients, of whom 92 (25%) had prior MDS and 38 (10%) had therapy-related AML. Diagnostic BM blasts were ≤30%, 31% to 50%, and >50% in 41%, 24%, and 29% of patients, respectively. The 1L therapy led to BM blast clearance in 54% of patients (115/215) overall and in 67% (38/57), 62% (68/110), and 19% (9/48) of Cohorts A, B, and C, with median durations of blast clearance of 6.3, 6.9, and 5.4 months, respectively. Only 30 patients (8%) received allo-HSCT, the majority of whom were from Cohort B (21 patients, 16%).
Death during follow-up was reported for 80% (n = 294) of patients, and mOS (95% CI) in all patients was 7.3 months (6.5–8.3). The mOS (95% CI) decreased with age: 9.5 months (7.2–12.1) among patients <65 years, 6.8 months (4.9–8.8) among those aged 65 to 74 years, and 6.7 months (5.3–8.2) among patients aged ≥75 years. Estimated mOS (95% CI) was 7.4 months (6.0–8.8) for Cohort A, 9.4 months (7.2–10.4) for Cohort B, and 5.9 months (4.3–7.5) for Cohort C (Figure 2). Patients who received allo-HSCT (n = 30, 8%) had longer mOS compared with those who did not (33.7 vs. 7.0 months). mOS (95% CI) with allo-HSCT was not reached in Cohort A (n = 5), was 28.9 months (12.8–36.5) in Cohort B (n = 21), and 45.5 months (44.1–46.9) in Cohort C (n = 4). Similarly, patients with del17p only had longer mOS compared with those who had only TP53m or both TP53m and del17p (13.2 vs. 8.3 vs. 6.8 months; Figure 2).
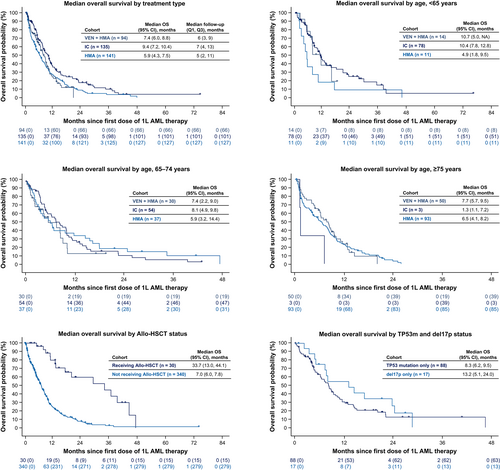
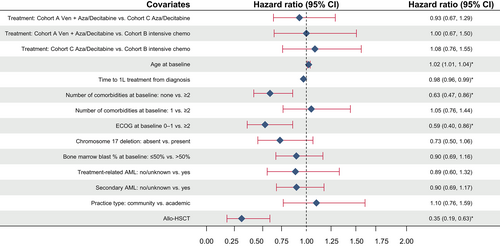
Addition of VEN to HMA (Cohort A) did not improve OS compared with HMA only (Cohort C; adjusted hazard ratio [aHR], 0.93 [95% CI, 0.7–1.3]) after adjusting for effects of relevant patient characteristics, commonly associated with outcome, using a multivariate Cox model. Age, time from diagnosis to 1L therapy initiation, number of comorbidities, ECOG at baseline, and treatment with allo-HSCT were significantly associated with survival among all patients in this model (Figure 3). Similarly, survival did not differ significantly when other treatment modalities were compared (VEN + HMA vs. IC, aHR [95% CI], 1.0 [0.7–1.5]; HMA vs. IC, aHR [95% CI], 1.1 [0.8–1.6]).
4 DISCUSSION
To our knowledge, this is among the first and largest retrospective cohort studies using data from a nation-wide real-world database to evaluate the OS of AML patients with TP53m receiving different SOC treatments. In this population of patients with AML along with TP53m and/or del17p, poor OS was observed across SOC treatment options, and OS did not differ significantly using different treatment modalities. Our study results demonstrate poor OS and are consistent with published findings for AML patients with TP53m.3, 5, 11, 25, 26
In a systematic literature review, treatment responses in TP53m AML populations were reported using similar inclusion and exclusion criteria.27 CR rates were reported from six trials and were highest in the IC treatment cohort, followed by VEN + HMA and HMA alone, while mOS (seven studies: six clinical trials and one retrospective observational study) was similarly low across the three treatment groups.27 The single retrospective observational study (N = 22) reported an mOS of 8.5 months for the IC cohort.28
In the current study, the apparent higher estimated OS in the IC cohort was likely due to differences in the populations treated: patients receiving IC were younger (median age 75, 62, and 77 for Cohorts A, B, and C, respectively), had fewer comorbidities (<2 comorbidities in 62%, 85%, and 70% of patients in Cohorts A, B, and C, respectively), and were more often bridged to allo-HSCT (5%, 16%, and 3% in Cohorts A, B, and C, respectively; mOS [95% CI] in Cohort B patients who received allo-HSCT was 28.9 [12.8 to 36.5] months). Indeed, after adjusting for relevant baseline characteristics and allo-HSCT treatment, no differences in OS were seen by type of treatment received—VEN + HMA, IC, or HMA only.
This study included AML patients who were diagnosed between 2014 and 2020. Approximately 30% of patients were diagnosed with TP53m AML before 2017, and these patients represent a subpopulation for whom VEN + HMA was not available as an option, as VEN was only approved by the US FDA as a treatment for AML in 2018. Despite patients receiving VEN + HMA at a later period, the median and interquartile range of follow-up time for the 3 treatment groups were similar (median follow-up of 6, 7, and 5 months for VEN + HMA, IC, and HMA groups, respectively; Table 1). The median OS for all 3 groups was <10 months. It is unlikely that the findings would be significantly influenced by these factors.
Although mortality data were collected from multiple sources, there could be missing death information in the dataset. Previous assessment of Flatiron data in non-small cell lung cancer suggests the OS could be overestimated due to missing death data, but also showed the impact of missing data to be minimal when mortality-capture sensitivity was high (e.g., approximately ≥90%).29 Flatiron data showed a sensitivity of 89% to mortality across 18 cancer types, so the influence of missing death data on OS could be minimal.23 In addition, this study required at least two clinic visits and allowed a 60-day gap after diagnosis for 1L therapy initiation and at least 3 months of follow-up. These factors could have selected patients more likely to survive to the start of 1L therapy and may have missed some very early deaths, thus potentially contributing to higher mOS estimates. Details about TP53m or del17p in patients, including whether they were monoallelic or biallelic, were also not available. As clonal burden and allelic involvement may contribute to outcomes in TP53m AML, as published by some groups,5 the findings from this study should be interpreted taking into consideration the absence of these data. It is possible that due to a large number of unknown/missing data on TP53m, patients with better prognostic profiles (such as monoallelic del17p without TP53m) were included in our analyses. Overall, among patients with only del17p, mOS (95% CI) was 13.2 (5.1–24.0) months, and among patients with only TP53m, mOS (95% CI) was 8.3 (6.2–9.5) months. For patients with both TP53m and del17p, mOS (95% CI) was 6.8 (5.4–9.6) months.
In a related comparison study of patients with solid tumors, data from the Flatiron Health database were generally representative of the US population in terms of demographics and regional distribution, although the Flatiron patient population tended to be younger than those in the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results registry. There was also substantial disease-dependent variability in capturing tumor-specific and clinical data across these three databases.30 Overall, the retrospective nature of the data along with the higher representation of patients from community settings conferred limitations in terms of data completeness, quality, and generalizability inherent to many real-world data.
This is among the largest observational studies to date that describe outcomes for TP53m AML patients. The dataset includes relevant molecular/cytogenetic data, longitudinal treatment, and clinical outcomes data with comprehensive quality assurance and quality control, and compares to national estimates. However, data on important variables were missing, such as complex karyotypes (not captured in the database), remission rates (not captured in the database), and cytogenetic risk categories (missing in 34% of patients). The magnitude and directionality of the effect of missing data on outcome estimates cannot be ascertained.
Immunotherapies, such as monoclonal antibodies, bispecific antibodies, and chimeric antigen receptor T-cell therapy have been widely used to treat malignancies by facilitating effector T-cell responses and utilizing adaptive immune checkpoints. These approaches are now being investigated for the treatment of AML, particularly in various subtypes, such as patients with TP53m.31-33 A challenge in treating AML is that, unlike other hematologic malignancies, such as acute lymphocytic leukemia and lymphoma, specific and differentially expressed antigens are more variable and often expressed on normal hematopoietic stem/progenitor cells and healthy organs, making the targeting of one antigen potentially insufficient.2
Eprenetapopt, a novel, first-in-class small molecule that induces TP53m cell apoptosis, is in development for the treatment of TP53m MDS or AML. A Phase 3 trial in TP53m MDS reported a CR rate of 33.3% with eprenetapopt + AZA compared with 22.4% with AZA alone but did not reach the pre-specified statistical significance (p = .13).34 A Phase 1 trial of eprenetapopt + AZA + VEN was initiated in TP53m AML and has reported encouraging early efficacy data.35
Cluster of differentiation 47 (CD47) is a widely expressed cell surface protein that binds signal regulatory protein alpha (SIRPα) on phagocytic cells and imparts an antiphagocytic “don't eat me” signal. Magrolimab is a first-in-class humanized immunoglobulin G4 anti-CD47 monoclonal antibody that blocks the CD47-SIRPα interaction to promote cancer cell phagocytosis.36-38 In a Phase 1b study in patients with AML ineligible for IC, magrolimab + AZA as 1L showed promising efficacy in those with TP53m; objective response rate (n = 72) was 48.6% (33.3% CR, 8.3% CRi, 5.6% partial response), and the mOS was 10.8 months.36 A Phase 3 trial (ENHANCE-2; NCT04778397) is ongoing and compares the safety and efficacy of magrolimab + AZA with SOC (VEN + AZA or IC) in adults with previously untreated TP53m AML.
5 CONCLUSION
Despite recent therapeutic advances in AML, the subgroup of AML patients with TP53m still experiences poor outcomes. These findings highlight the unmet need for treatment modalities for patients with TP53m AML and the opportunity for the development of novel therapeutic approaches. The anticipation of developmental-stage therapies that specifically target TP53m AML patients and offer significant improvement in response and/or OS is high, and so far, the results are encouraging.
FUNDING INFORMATION
This study was funded by Gilead Sciences, Inc.
CONFLICT OF INTEREST STATEMENT
NGD has received grants or contracts from Hanmi, Trovagene, Fate Therapeutics, Novimmune, and GlycoMimetics; consulting fees from Arog, Novartis, Jazz, Celgene, Syndax, Shattuck Labs, and Agios; and grants or contracts and consulting fees from Daiichi-Sankyo; Bristol-Meyers Squibb; Pfizer; Gilead Sciences, Inc.; Servier; Genentech; Astellas; AbbVie; ImmunoGen; Amgen; and Trillium. SI, JH, CR, JL, YP, MW, and GR are employees and stockholders of Gilead Sciences, Inc. CR is an Alphabet stockholder.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study have been originated by Flatiron Health, Inc. Requests for data sharing by license or by permission for the specific purpose of replicating results in this manuscript can be submitted to [email protected].




