The Gut Microbiome in Aging and Ovarian Cancer
Funding: This research was funded by National Institutes of Health/National Cancer Institute (Grants RO1CA109545 [M.S.S.] and UO1CA236979 [M.S.S.]), the Samuel Waxman Cancer Research Foundation (M.S.S.), and the Dolores Zohrab Liebmann Fund (G.M.D.).
ABSTRACT
The gut microbiome changes with age and affects regions beyond the gut, including the ovarian cancer tumor microenvironment. In this review summarizing the literature on the gut microbiome in ovarian cancer and in aging, we note trends in the microbiota composition common to both phenomena and trends that are distinctly opposite. Both ovarian cancer and aging are characterized by an increase in proinflammatory bacterial species, particularly those belonging to phylum Proteobacteria and genus Escherichia, and a decrease in short-chain fatty acid producers, particularly those in Clostridium cluster XIVa (family Lachnospiraceae) and the Actinobacteria genus Bifidobacterium. However, although beneficial bacteria from family Porphyromonadaceae and genus Akkermansia tend to increase with normal, healthy aging, these bacteria tend to decrease in ovarian cancer, similar to what is observed in obesity or unhealthy aging. We also note a lack in the current literature of research demonstrating causal relationships between the gut microbiome and ovarian cancer outcomes and research on the gut microbiome in ovarian cancer in the context of aging, both of which could lead to improvements to ovarian cancer diagnosis and treatment.
1 Introduction
An emerging field within cancer research is the impact of the gut microbiota on cancer progression and treatment. The gut microbiota composition has been found to differ in cancer patients and healthy control subjects, impact carcinogenesis, and modulate the effectiveness of cancer therapies [1, 2]. Although the term “gut microbiome” can be expanded, most gut microbiome studies focus on the bacterial taxa present in the colon with the genes and metabolites relevant to the topic at hand [3]. Many studies address how the gut microbiota directly affects the tumor microenvironment, as seen in colorectal cancer, where the microbiota are in physical contact with the tissue from which the tumors arise. However, researchers have also begun to ask how the gut microbiome affects the tumor microenvironment of malignancies like ovarian cancer, which neither originate in nor metastasize to the gut.
1.1 Ovarian Cancer: Mechanism of Metastasis and Prognosis
Ovarian cancer generally metastasizes intraperitoneally as single cells or multicellular aggregates, which detach from the primary lesion, migrate directly through the peritoneal fluid, and attach to the mesothelial lining of the abdominal organs to form secondary tumors [4-6]. Ascites fluid builds up in the peritoneal cavity as the cancer causes blood vessel leakiness and blocks lymphatic vessels, leading to increased abdominal distension [4, 7]. Metastatic tumors fully colonize the omentum, compressing the gastrointestinal tract and causing bowel obstruction [4]. The 5-year survival rate of ovarian cancer is below 50% and even lower for minorities, making ovarian cancer the most fatal malignancy of the female reproductive system and the fifth largest cause of death due to cancer in women [8]. The low rate of ovarian cancer survival is related to a lack of early diagnoses, resulting in patients that initially present with more advanced stages of cancer and have fewer treatment options [9, 10], recurrence, which occurs in over 80% of patients diagnosed with advanced disease [11], and age, which causes structural changes to the collagen, adipose tissue, and immune cell populations of the peritoneal cavity where ovarian cancer metastasizes [12]. Ovarian cancer is predominantly diagnosed in older women, with 63 being the median age at diagnosis and 71 being the median age at death [13].
With a large proportion of ovarian cancer patients approaching old age by the time the cancer becomes apparent, it is important to consider the impact of aging when evaluating the gut microbiome in ovarian cancer. The composition, diversity, and functions of the gut microbiome change as an individual ages, often negatively impacting metabolism, systemic inflammation, and the immune response, all of which are implicated in ovarian cancer risk, treatment response, and survival [14, 15]. This review will examine the intersection between aging, the bacterial components of the gut microbiome, and ovarian cancer, highlighting the need to consider aging in research on the gut microbiome in ovarian cancer. In order to aid in reviewing the literature covering the gut microbiome in both aging and ovarian cancer, the study designs of the key papers cited in this review are summarized in Table 1.
| Featured topic | Description | Reference |
|---|---|---|
| Gut microbiome of ovarian cancer patients compared to control subjects | Fecal samples collected prior to treatment of 40 ovarian cancer patients and 40 patients exhibiting benign disease of the ovary were analyzed by 16S rRNA sequencing to determine microbial signatures of ovarian cancer. The samples were used for FMT in mice injected intraperitoneally with syngeneic ovarian cancer cells to demonstrate the impact of the ovarian cancer gut microbiome on ovarian cancer progression | [16] |
| Pre-treatment fecal samples of ovarian cancer patients, patients exhibiting benign disease of the ovary, and healthy controls (n = 20 each) were analyzed by 16S rRNA sequencing to determine microbial signatures of ovarian cancer. The samples were used for FMT in mice injected subcutaneously with syngeneic ovarian cancer cells to demonstrate the impact of the ovarian cancer gut microbiome on ovarian cancer progression | [17] | |
| Fecal samples of 24 ovarian cancer patients and 24 healthy control subjects were compared prior to treatment. Samples were also taken from the ovarian cancer patients over the course of treatment. All samples were analyzed with 16S rRNA sequencing | [18] | |
| Microbiota samples from the reproductive tract, peritoneal cavity, and urine were taken from patients undergoing hysterectomy for ovarian cancer (n = 34) or a benign gynecological condition (n = 30). Stool samples were taken from the ovarian cancer patients, differentiated by stage (early, n = 7; advanced, n = 27). All microbiota samples were analyzed by 16S rRNA sequencing to identify disease signatures | [19] | |
| Gut and vaginal microbiota samples from 40 ovarian cancer patients and 5 patients with benign gynecological conditions were analyzed by 16S rRNA sequencing to determine microbiome differences in patients with ovarian cancer vs. benign disease or short vs. long platinum-free intervals | [20] | |
| Antibiotic use in ovarian cancer patients | 424 ovarian cancer patients were compared retrospectively to determine if >48 h of antibiotic treatment during chemotherapy affects survival | [21] |
| Antibiotic use in mouse models of ovarian cancer | Antibiotic treated or control mice were injected intraperitoneally with syngeneic ovarian cancer cells and treated with cisplatin to determine the impact of gut microbiota disruption with antibiotics on platinum resistance and survival | [22] |
| Mice injected subcutaneously with syngeneic ovarian cancer cells were treated with oral antibiotics for 3 days to induce gut dysbiosis and treated with cisplatin for 14 days. The effects of gut dysbiosis on tumor growth, cisplatin sensitivity, and cancer signaling pathways were compared to saline-treated controls | [23] | |
| Gut microbiome and ovarian cancer chemotherapy in human subjects | Fecal samples from 12 ovarian cancer patients undergoing adjuvant chemotherapy and 12 undergoing neoadjuvant chemotherapy were collected over the course of chemotherapy and compared via 16S rRNA sequencing to identify changes occurring with ovarian cancer therapy | [18] |
| Fecal samples collected at diagnosis and over the course of cytoreductive surgery and chemotherapy for 18 ovarian cancer patients were compared via 16S rRNA sequencing to identify changes occurring with ovarian cancer therapy | [24] | |
| Gut microbiome and ovarian cancer chemotherapy in mouse models | Gut microbiota from mice injected intraperitoneally with syngeneic ovarian cancer cells and treated with cisplatin were analyzed by 16S rRNA sequencing to determine compositional differences caused by cisplatin treatment. Since Ruminococcus gnavus and gut mucus thickness were depleted by cisplatin, ovarian cancer bearing cisplatin-treated mice were supplemented with R. gnavus or FMT from tumor bearing mice prior to cisplatin treatment to restore the gut mucus layer | [25] |
| Gut microbiome and normal aging in humans | Gut microbiota samples from 1550 healthy Ukrainian subjects aged 0 to 69 were compared at the phylum level. Samples from subjects older than 70 were collected but not reported with the overall analysis due to the lower number of subjects | [26] |
| Fecal samples from 161 65+ year and 9 young adult Irish subjects were analyzed by 16S rRNA sequencing to determine the variability and temporal stability of the elderly human gut microbiome | [27] | |
| Gut microbiota of Italian centenarians (n = 21), elderly subjects unrelated to the centenarians (n = 22), elderly offspring of the centenarians (n = 21), and young adults (n = 20) were compared using HITChip and 16S rRNA sequencing to determine changes in the gut microbiome with age and longevity | [28] | |
| Gut microbiota from 367 Japanese subjects aged birth to 104 years were compared to determine common changes in the gut microbiome with age | [29] | |
| Gut microbiota from 531 subjects aged 0–86 years from Venezuela (n = 100), Malawi (n = 115), and the United States (n = 316) were compared to determine common changes in the gut microbiome with age | [30] | |
| Gut microbiome and mortality data of 9000+ subjects aged ∼18–98 from 3 cohorts were analyzed for microbiome signatures to predict survival in aging patients | [31] | |
| Gut microbiota deep metagenomic sequencing, dietary, and clinical chemistry data of 1098 subjects aged 18–65 years from the United States and the United Kingdom were compared to identify associations between gut microbiota, dietary food patterns, and overall health | [32] | |
| Gut microbiome and unhealthy aging in humans | Gut microbiota profiles of elderly Irish subjects living in the community (n = 83), attending an outpatient day-hospital (n = 20), in short-term rehabilitation (n = 15), or in long-term residential care (n = 60) were compared by 16S rRNA sequencing to determine the role of living situation and diet on gut microbiome signature in the elderly | [33] |
- Abbreviation: FMT, fecal microbiota transplant.
2 The Gut Microbiome in Ovarian Cancer
It is possible to experimentally demonstrate direct relationships between the gut microbiome and ovarian cancer outcomes. Mice-injected intraperitoneally with syngeneic ovarian cancer cells and given fecal microbiota transplant (FMT) from ovarian cancer patients had an increased rate of tumor growth compared to mice given FMT from patients exhibiting benign disease of the ovary [16]. In another study, mice bearing subcutaneous syngeneic ovarian cancer tumors exhibited Hedgehog and TNF-α/IL-1β signaling above the tumor-bearing baseline in response to FMT from ovarian cancer patients, but signaling from these pathways dropped below the baseline and tumor growth decreased when the mice were given FMT from healthy control subjects [17]. However, functional studies are rare, and retrospective studies on human subjects and experimental research on animal models (mainly murine) are much more common. These studies in ovarian cancer patients and mice have highlighted several specific gut microbiome characteristics that affect or are affected by ovarian cancer and ovarian cancer treatment.
2.1 Alpha Diversity
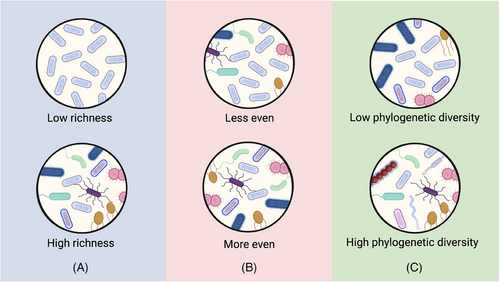
Alpha diversity is an ecological term accounting for the diversity of taxa in an ecosystem, in this case, the bacteria in the gut of each human or model animal subject. The simplest measure of alpha diversity is richness, that is, the number of unique taxa (species, genera, etc.) present in the ecosystem, but there are also alpha diversity indices accounting for the evenness and phylogenetic diversity of the bacteria present. Figure 1 illustrates these concepts in the context of bacterial populations. In this article, “alpha diversity” without qualifiers refers to observations occurring in several alpha diversity measures, whereas qualifiers, such as richness, evenness, or phylogenic diversity, will be used when this particular aspect of alpha diversity is of importance. In the absence of factors that have antimicrobial activity, such as chemotherapy and antibiotic use, the alpha diversity of the ovarian cancer gut microbiome is similar to that of control populations. An Italian study comparing ovarian cancer patients (n = 24) at the time of diagnosis to demographically matched healthy controls (n = 24) noted similar alpha diversity between the two groups [18]. These findings were corroborated in a rat model of ovarian cancer metastasis, where the gut alpha diversity of rats injected intraperitoneally with syngeneic ovarian cancer cells did not differ significantly from that of saline-injected rats [34]. Lack of significant difference in the gut microbiome is also seen when comparing ovarian cancer patients to groups other than healthy subjects. A Chinese study sampling ovarian cancer patients (n = 40) and patients with benign disease of the ovary (n = 40) found extremely similar gut alpha diversity between the ovarian cancer patients and the benign controls [16]. An American study likewise found that chemotherapy-free participants with benign gynecological disease (n = 5) had similar alpha diversity to chemotherapy-treated ovarian cancer patients (n = 40). This study also found no difference in alpha diversity between platinum-sensitive (>24 months without recurrence, n = 23) and platinum-resistant (<6 months before recurrence, n = 17) ovarian cancer patients sampled after chemotherapy [20]. Finally, another American study also found no significant differences in the alpha diversity based on the stage of ovarian cancer prior to chemotherapy: early (n = 7) or advanced (n = 27) [19]. However, one Chinese study did find significantly lower alpha diversity in ovarian cancer patients prior to chemotherapy (n = 20), particularly those with advanced disease, compared to healthy control subjects (n = 20) [17]. Though this study found significant differences in alpha diversity, four human studies sampling from three cultural and geographical regions, as well as one rat study, did not find significant differences in alpha diversity when comparing subjects of different ovarian cancer stage or presence, suggesting that the factors at play between the gut microbiome and ovarian cancer rely more on the species composition than in overall diversity.
2.2 Estrogen Metabolism
The influence of the gut microbiome on circulating estrogen has been reviewed extensively elsewhere [35-39]. Gut dysbiosis can lead to obesity and imbalanced estrogen levels, both of which are risk factors for ovarian cancer [36]. Several species, particularly from the Firmicutes taxa Ruminococcaceae and the Clostridium coccoides and Clostridium leptum groups, as well as the Actinobacterium genus Bifidobacterium, produce β-glucuronidase, which deconjugates estrogens secreted into the bile, allowing them to be reabsorbed and bind to estrogen receptors. Furthermore, a significant correlation has been found between urine estrogen levels (a proxy for circulating estrogen) and the gut microbiota species richness, both in postmenopausal women and in men [38].
2.3 Taxa Affected by Ovarian Cancer
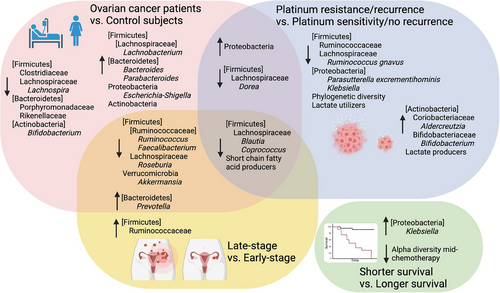
There are several compositional differences in gut bacteria that have been observed in ovarian cancer. The bacterial component of the gut microbiome is dominated by phylum Firmicutes, which is generally followed by Bacteroidetes, with variable proportions of Proteobacteria and Actinobacteria depending on age and disease state. Phylum Verrucomicrobia accounts for a small portion of the gut microbiota but contains Akkermansia muciniphila, which is attracting increased attention due to its positive role in protecting the intestinal mucus layer. The important taxa affected by ovarian cancer are summarized in Figure 2 and Table 2. Phylum Firmicutes generally comprises about 60% of the gut microbiome in healthy, pre-elderly adults, though the composition can range from 40% to 80% depending on the study population, whereas phylum Bacteroidetes is generally second to Firmicutes, constituting about 30% ± 10% of the gut microbiome. However, the sum of Firmicutes and Bacteroidetes almost always constitutes around 90% of the gut microbiome in healthy pre-elderly adults [26, 40, 27-29]. Firmicutes and Bacteroidetes contain a diverse array of species, making it difficult to pinpoint a trend for these bacteria at the phylum level in ovarian cancer patients. Nonetheless, several trends can be seen with respect to lower taxa.
| Taxon | Direction | Details | Reference |
|---|---|---|---|
|
P. Firmicutes C. Clostridia O. Clostridiales F. Clostridiaceae |
Down | Decreased family Clostridiaceae in ovarian cancer patients at diagnosis compared to healthy controls | [18] |
|
P. Firmicutes C. Clostridia O. Clostridiales F. Lachnospiraceae |
Down | Decreased family Lachnospiraceae in ovarian cancer patients at diagnosis compared to healthy controls | [18] |
| Down | Decreased genus Dorea in ovarian cancer patients:
|
[18, 19] | |
| Down | Decreased genus Coprococcus in ovarian cancer patients:
|
[17, 18] | |
| Down | Decreased genus Lachnospira in ovarian cancer patients at diagnosis compared to healthy controls | [18] | |
| Down | Decreased genus Roseburia in ovarian cancer patients:
|
[17, 18] | |
| Down | Decreased genus Blautia in ovarian cancer patients:
|
[16-19] | |
| Down | Decreased genus Lachnoclostridium in ovarian cancer patients with adverse cancer events 2 years post-sampling compared to patients with no adverse events at that time | [19] | |
| Up | Increased genus Lachnobacterium in ovarian cancer patients at diagnosis compared to healthy controls | [18] | |
| Down | Decreased Ruminococcus gnavus in ovarian cancer patients with adverse cancer events 2 years post-sampling compared to patients with no adverse events at that time | [19] | |
|
P. Firmicutes C. Clostridia O. Clostridiales F. Ruminococcaceae |
Up | Increased family Ruminococcaceae was in late-stage vs. early-stage ovarian cancer | [19] |
| Down | Family Ruminococcaceae was negatively associated with platinum resistance | [18] | |
| Down | Decreased genus Ruminococcus in ovarian cancer patients:
|
[17] | |
| Down | Decreased genus Faecalibacterium in ovarian cancer patients:
|
[17] | |
| Up | Increased genus Faecalibacterium in ovarian cancer patients compared to patients with benign gynecological conditions | [16] | |
|
P. Bacteroidetes C. Bacteroidia O. Bacteroidales |
Down | Decreased family Porphyromonadaceae in ovarian cancer patients at diagnosis compared to healthy controls | [18] |
| Down | Decreased family Rickenellaceae in ovarian cancer patients at diagnosis compared to healthy controls | [18] | |
| Up | Increased genus Bacteroides in ovarian cancer patients:
|
[17] | |
| Up | Increased genus Parabacteroides in ovarian cancer patients compared to healthy controls | [17] | |
| Up | Increased genus Prevotella in ovarian cancer patients:
|
[17, 19, 20] | |
| P. Proteobacteria | Up | Increased Proteobacteria in ovarian cancer patients:
|
[16-18] |
|
P. Proteobacteria C. Gammaproteobacteria |
Up | Increased genus Escherichia-Shigella in ovarian cancer patients compared to patients with benign gynecological conditions | [16] |
| Up | Increased Pseudomonas in ovarian cancer patients with:
|
[24] | |
|
P. Proteobacteria C. Betaproteobacteria |
Down | Decreased Parasutterella excrementihominis in ovarian cancer patients who experienced adverse cancer events 2 years after sampling compared to patients with no adverse events at that time | [19] |
|
P. Actinobacteria, C. Coriobacteriia O. Coriobacteriales F. Coriobacteriaceae |
Up | Increased family Coriobacteriaceae in ovarian cancer patients at diagnosis compared to healthy controls | [18] |
| Up | Increased genus Adlercreutzia in ovarian cancer patients at the time of diagnosis compared to healthy controls, discriminating factor for platinum resistance | [18] | |
| Up | Increased genus Collinsella in ovarian cancer patients at diagnosis compared to healthy controls, discriminating factor for platinum resistance | [18] | |
|
P. Actinobacteria C. Actinobacteria O. Bifidobacteriales F. Bifidobacteriaceae |
Down | Decreased genus Bifidobacterium in ovarian cancer patients:
|
[16-18] |
| Up | Increased genus Bifidobacterium in ovarian cancer patients with:
|
[18, 24] | |
| P. Verrucomicrobia | Down | Decreased Verrucomicrobia in ovarian cancer patients with late vs. early-stage disease | [17] |
| Down | Decreased genus Akkermansia in ovarian cancer patients:
|
[16, 17, 34] | |
|
P. Firmicutes C. Negativicutes O. Veillonellales-Selenomonadales F. Selenomonadaceae |
Up | Increased genus Megamonas in ovarian cancer patients with:
|
[24] |
The changes in Firmicutes are concentrated in the families Clostridiaceae, Lachnospiraceae, and Ruminococcaceae. All three of these families contain short-chain fatty acid (SCFA)-producing bacteria, which are generally beneficial because SCFAs reduce inflammation [41]. The overwhelming trend for key genera in families Clostridiaceae and Lachnospiraceae is to decrease with ovarian cancer presence, stage, recurrence, and metastasis [16-19, 24]. Family Ruminococcaceae cannot be discussed without nuance as its members appear to follow different patterns in ovarian cancer depending on the comparison. First, it should be noted that genus Ruminococcus includes species that have been assigned to several families in class Clostridia, mainly Lachnospiraceae and Ruminococcaceae [42]. Changes in Ruminococcus species that fall under different families are discussed with their respective families in this review. Gut microbes from family Ruminococcaceae have been found to be 500-fold higher in late-stage versus early-stage ovarian cancer [19]. Genus Faecalibacterium or the species F. prausnitzii were decreased in ovarian cancer patients, particularly those diagnosed with late-stage disease, compared to healthy controls [17], though increased in ovarian cancer patients compared to patients with benign disease of the ovary [16].
The increased Ruminococcaceae in patients with late-stage ovarian cancer at diagnosis may be reflective of the role of Ruminococcaceae members in estrogen reabsorption [38]. Although one study showed increased Ruminococcaceae in late- compared to early-stage ovarian cancer [19], some Ruminococcaceae Ruminococcus species were decreased in another cohort of ovarian cancer patients with late- compared to early-stage ovarian cancer [17]. However, because the two sources mentioning changes to family Ruminococcaceae in late- versus early-stage ovarian cancer do not list particular species, it is unclear whether there are contradictory results for the same set of species or if this discrepancy is merely due to different Ruminococcaceae species. Similarly, in the case of the reported decrease in Faecalibacterium in ovarian cancer patients relative to healthy controls but increase in Faecalibacterium in ovarian cancer patients relative to controls with benign disease, it is unclear whether this represents a true discrepancy in the direction of change in Faecalibacterium with ovarian cancer or whether patients with benign disease have a lower relative abundance of Faecalibacterium than both healthy subjects and ovarian cancer patients. Finally, it may be that the dual roles of producing SCFAs and promoting estrogen reabsorption render Ruminococcaceae members capable of helping or hurting ovarian cancer patients, depending on the species and situation.
Bacteria from phylum Bacteroidetes are important Gram-negative commensals, specializing in the digestion of proteins and carbohydrates [43]. Most changes in Bacteroidetes with ovarian cancer appear in order Bacteroidales. The Bacteroidales families Porphyromonadaceae and Rikenellaceae were decreased in ovarian cancer patients at the time of diagnosis compared to healthy controls in one 24-patient cohort [18]. Porphyromonadaceae and Rikenellaceae have been negatively associated with obesity, a major risk factor for ovarian cancer, so their decreased levels in ovarian cancer patients may reflect one of the pathways through which obesity promotes the disease [44, 45]. Genera Bacteroides, Parabacteroides, and Prevotella from other Bacteroidales families were increased in ovarian cancer patients compared to healthy control subjects in another study with 20 patients [17]. Prevotella was also significantly higher in ovarian cancer patients compared to patients with benign disease [20], and Prevotella buccalis was increased about 1000-fold in late-stage versus early-stage ovarian cancer patients [19]. Prevotella, though commonly found in the gut microbiome, is a known mediator of T helper type 17 (TH17) cell-mediated inflammation above the level of other gut commensals [46]. TH17 cells have a dichotomous role in cancer because their ability to promote an anti-tumor immune response is coupled with ability to promote angiogenesis and immunosuppressive signaling [47, 48]. However, more research on the specific effects of Prevotella species in ovarian cancer would be needed to establish a connection between the increased Prevotella found in the studies reviewed here.
Proteobacteria, a phylum containing several pathogenic or disease-associated species, tend to occupy a larger percentage of the microbiome with ovarian cancer [16-18, 49]. However, specific mechanisms of Proteobacteria in ovarian cancer have not yet been established. There are no clear trends with ovarian cancer in phylum Actinobacteria except that family Bifidobacteriaceae and genus Bifidobacterium were decreased in ovarian cancer patients at the time of diagnosis compared to healthy controls or patients with benign disease in three separate studies [16, 17, 19]. Bifidobacterium is an SCFA producer that is generally positively associated with health and negatively associated with obesity, so its decrease in ovarian cancer is not surprising [41].
Phylum Verrucomicrobia is a minor phylum in the gut microbiome but has a prominent place in the literature on the gut microbiome in ovarian cancer. Akkermansia, the primary Verrucomicrobia genus found in the human gut, is a major player in maintaining the gut mucus layer and balance of beneficial bacterial species [41]. Verrucomicrobia and Akkermansia were decreased in patients with advanced compared to early-stage ovarian cancer [17]. One study found that Akkermansia was decreased in the gut of ovarian cancer patients compared to patients with benign disease and that supplementing ovarian cancer-injected mice with Akkermansia slowed tumor growth, provided the mice had also been given FMT from ovarian cancer patients. This group further confirmed that the main benefit to Akkermansia was increased fecal acetate levels, which promoted CD8+ T-cell cancer-killing ability via IFN-γ [16]. Another study on rats injected with syngeneic ovarian cancer cells found decreased Akkermansia in the tumor-bearing rats compared to the tumor-naïve rats [34].
2.4 Effects of Antibiotics on Ovarian Cancer Treatment
Antibiotic use during ovarian cancer treatment is commonly employed to treat or prevent infections following cytoreductive surgery or during platinum chemotherapy, but this life-saving treatment may ultimately reduce ovarian cancer survival. A retrospective study of 424 women receiving cytoreductive surgery and platinum chemotherapy for high-grade epithelial ovarian cancer included 147 patients (34.7%) who received >48 h of antibiotic treatment during chemotherapy. Recurrence was significantly higher and progression-free, and overall survival significantly lower in patients receiving antibiotics compared to no antibiotics, and in patients receiving anti-Gram-positive antibiotics compared to other antibiotics during chemotherapy [21]. This result has been corroborated in a retrospective study of human patients with relapsed lymphoma, which found that concurrent anti-Gram-positive antibiotics with platinum chemotherapy significantly reduced overall response and survival [50]. Another group has shown in mice bearing subcutaneous tumors from three types of cancer that gut leakiness induced by platinum chemotherapy enhanced treatment by allowing Gram-positive bacteria to leave the gut, enter the lymph nodes, and activate tumor-infiltrating myeloid cells via Toll-like receptors, providing a putative explanation for the added detriment of anti-Gram-positive antibiotics [51].
Murine ovarian cancer studies have shown detrimental effects of antibiotics to ovarian cancer outcomes, depending on the stage of cancer being studied. Antibiotic-treated mice injected hypodermically in the lower abdomen with human xenograft ovarian cancer cells had higher tumor burden and increased epithelial-to-mesenchymal transition compared to their non-antibiotic treated counterparts [52]. Disrupting the gut microbiome of mice with antibiotics 2 weeks prior to intraperitoneal ovarian cancer cell injection and throughout the course of cisplatin chemotherapy accelerated tumor growth, increased cancer stem cells, and decreased survival in both immunocompetent and immunocompromised mice, indicating that a fully functional immune system is not required to see detrimental effects of antibiotics. These immunocompetent mice also exhibited increased angiogenesis and decreased sensitivity to cisplatin when treated with antibiotics, and when treated with cecal microbiota transplant from either antibiotic-treated or untreated tumor-naïve mice, they exhibited tumor burden and cisplatin sensitivity comparable to those of the microbiota donor [22]. Similar results were documented in another report in which mice receiving cisplatin, Tripterygium glycosides, and oral antibiotics had greater subcutaneous ovarian tumor growth than mice given the same treatment without antibiotics. FMT from tumor-naïve donor mice reduced tumor growth in the antibiotic-treated mice to levels comparable to those not given antibiotics [23]. Because both the retrospective human study and these murine studies indicate negative effects of antibiotics on ovarian cancer metastasis and treatment, it will be important to further validate this research and carefully weigh the benefits and detriments of antibiotic use in ovarian cancer patients, especially during treatment.
2.5 Effects of Ovarian Cancer Treatment on the Gut Microbiome
The standard of care for ovarian cancer is cytoreductive surgery followed by platinum and taxane chemotherapy, usually carboplatin or cisplatin with paclitaxel for five to six cycles. In cases of heavy tumor burden at the time of diagnosis, chemotherapy may both precede and follow cytoreductive surgery if conditions are not optimal for an initial debulking. A study of 18 ovarian cancer patients receiving cytoreductive surgery followed by cisplatin or carboplatin with paclitaxel provides the most comprehensive account to date of the effects of cytoreductive surgery on the gut microbiome [24]. However, there is not adequate evidence to draw any conclusions as the report does not specify whether antibiotics were used in conjunction with the surgery, and pre- and post-operative fecal samples were obtained from only a subset of the eighteen patients (10 preoperative samples and 4 postoperative samples). On the other hand, there is sufficient evidence that chemotherapy affects the gut microbiome, and the gut microbiome affects chemotherapy outcomes, particularly with respect to platinum sensitivity and recurrence.
Platinum chemotherapy is known to induce gut dysbiosis and cause downstream adverse effects, including bacteremia and inflammation, due to damage to the intestinal mucosa [25]. Tumor-naïve mice treated with cisplatin showed cardiac dysfunction, weight loss, and increased expression of inflammatory genes, though Lactobacillus supplementation reversed these undesirable effects of cisplatin [53]. Mice injected intraperitoneally with syngeneic ovarian cancer cells and treated with cisplatin had increased members of the proinflammatory Bacteroidaceae family, particularly Bacteroides uniformis, with a decrease in the mucosa-supporting species Ruminococcus gnavus in the gut. Gavaging these mice with R. gnavus stimulated Muc3 mRNA production and infiltration of CD11b+ macrophages to the ileum, two steps in the restoration of the gut mucosa, but did not restore the cisplatin-induced weight loss or bacteremia. However, gavaging the mice with a fecal microbiota suspension collected from the same mice after ovarian cancer cell injection but prior to the beginning of cisplatin treatment partially restored the intestinal mucus layer and reduced the cisplatin-induced weight loss and bacteremia with its associated immune response [25]. In addition to demonstrating the necessity of whole gut microbiome health for the health of the intestinal mucus layer, these results set a precedent for FMT using one's own feces banked after cancer diagnosis but prior to chemotherapy.
Two small studies tracking the gut microbiota composition in ovarian cancer patients over the course of platinum chemotherapy found that some taxa were affected in the earlier cycles and returned to initial levels by later cycles, whereas other taxa required several cycles of chemotherapy to change in composition. However, there is almost no overlap in the taxa changing with chemotherapy between the two studies except for an increase in the Ruminococcaceae genus Faecalibacterium with later cycles of chemotherapy. The strength of the two studies is their comparison of gut microbiomes based on treatment response. The first study is the Chinese study mentioned with respect to cytoreductive surgery [24]. Eighteen ovarian cancer patients were treated with cytoreductive surgery, followed by six cycles of either carboplatin or cisplatin with paclitaxel. This study revealed a general decrease in conventionally “beneficial” bacteria and a general increase in potentially pathogenic bacteria with chemotherapy and noted significantly increased Bifidobacterium, Megamonas, and Pseudomonas in patients with shorter survival, corresponding to recurrence in less than 1 year following cessation of chemotherapy.
The second, an Italian study (n = 12 receiving surgery followed by carboplatin + paclitaxel; n = 12 receiving carboplatin + paclitaxel followed by surgery followed by additional carboplatin + paclitaxel) considered changes in the gut microbiome over the course of chemotherapy while differentiating patients by the time of recurrence (platinum-resistant = recurrence <6 months after the last cycle of chemotherapy; platinum-sensitive = recurrence >6 months after the last cycle of chemotherapy). The taxonomic signatures of platinum resistance are included in Table 2 for comparison with general gut microbiome differences observed in ovarian cancer. This study found that the platinum-resistant patients underwent more chemotherapy-induced fluctuation in gut microbiome composition than the platinum-sensitive patients, resulting in signatures of platinum resistance that were not significant before treatment and only became apparent in the later stages of chemotherapy [18].
Although the literature points to no differences in alpha diversity in ovarian cancer patients at diagnosis compared to control subjects, the Italian chemotherapy study saw reduced alpha diversity in all patients for the first few cycles of chemotherapy, with alpha diversity troughing after the middle cycle for each treatment group. A strong recovery of alpha diversity was observed in those with platinum-sensitive tumors, whereas reduced alpha diversity tended to persist in those with platinum-resistant tumors. Most notably, the gut alpha diversity of platinum-resistant patients was significantly lower than the baseline after the sixth cycle of chemotherapy, regardless of treatment group, and alpha diversity higher than the median at the midpoint of chemotherapy was non-significantly associated with longer survival for all groups [18]. These observations on alpha diversity and greater chemotherapy-induced instability are similar to the findings of another small study comparing the gut microbiome of ovarian cancer patients at the time they were diagnosed as either platinum resistant (<6 months without recurrence after initial carboplatin/paclitaxel treatment, n = 17) or platinum super-sensitive (>24 months without recurrence after initial treatment, n = 23). Specifically, platinum-resistant patients had higher odds of having an outlier gut microbiome profile, and the nine platinum-resistant patients who did have outlier microbiomes compared to the rest of the cohort also had significantly lower phylogenetic diversity [20].
The Chinese and Italian studies did not share a list of species altered by chemotherapy, perhaps due to their different geographical and cultural contexts. However, they did reveal a potential theme of gut microbiota-mediated excesses in lactate in platinum resistance and recurrence. Of the three genera increased in participants of the Chinese study who experienced recurrence in less than 1 year, two, Bifidobacterium and Megamonas, are implicated in lactate production [18, 24, 54], whereas one of the genera decreased with recurrence was Fusobacterium, which contains some strains capable of using lactate as a sole energy source [55]. The most distinctive taxonomic signature of platinum resistance (recurrence in <6 months) in the Italian study was higher proportions of Bifidobacterium and other lactate-producing bacteria, especially from the Actinobacteria family Coriobacteriaceae, whereas the platinum-sensitive patients had increased lactate utilizers from Firmicutes family Veillonellaceae [18]. The authors of the Chinese paper did not comment on the metabolism of the bacteria overrepresented with recurrence, but the authors of the Italian paper found many differences between the gut microbiota of platinum-resistant and platinum-sensitive patients with respect to both anabolic and catabolic pathways involving lactate. Thus, the authors of the Italian paper speculate that an excess of lactate-producing bacteria without sufficient lactate-utilizing bacteria was promoting the Warburg effect in the platinum-resistant patients [18]. Finally, the increased Bifidobacterium with platinum resistance may also be connected with estrogen reabsorption as this genus expresses β-glucuronidase, increasing circulating estrogen levels and driving ovarian cancer [35].
3 The Gut Microbiome in Aging
The gut microbiome undergoes many changes over the lifetime of an individual. There is increasing evidence that initial colonization of the gut begins in utero, though the route of microbial transmission to fetal tissue is currently unclear [56]. Further colonization of the gut occurs during birth, with the route of delivery affecting the composition of the gut microbiome for up to 4 years after birth [57, 58]. Following initial colonization, the most dramatic shift in the gut microbiota composition occurs during the transition from infancy to early childhood, with most individuals attaining an adult-like composition by age three [26]. A second major but more gradual shift occurs between middle and old age, and further major changes can occur in cases of extreme age [26, 29, 59]. Overall microbiome changes with age are distinctive enough that an unsupervised analysis of the gut microbiota from a cohort of 367 Japanese subjects from birth to 104 years was able to group the samples into four age categories with 100% accuracy [60]. Age-related changes in the gut microbiome affect health, as evidenced by the findings that intestinal dysbiosis is a predictor of the onset of health decline and death in fruit flies [61]. Additionally, germ-free mice do not experience age-related inflammation unless they are housed with aged, but not young, specific pathogen-free mice [62]. Intraperitoneal injection of young or aged mice with fecal slurries from aged mice also results in a worse septic response than injection with fecal slurries from young mice due to increased expression of virulence factors in the aged gut microbiota rather than age-related compositional differences [63]. Finally, mice receiving FMT from high-functioning elderly human donors showed improved grip strength compared to mice receiving FMT from low-functioning donors [64].
3.1 Alpha Diversity
Gut microbiota alpha diversity generally increases with normal and healthy aging. There are studies noting a decrease of alpha diversity with age, but these observations are accompanied by pathological conditions [26, 65-67]. The Japanese study showed alpha diversity increasing from birth to age 20, remaining relatively stable through adulthood, and increasing again from approximately 70 to 90 years of age [29]. A cohort of 531 participants from the United States, Malawi, and Venezuela exhibited a slight increase in alpha diversity with age in the adult and elderly years, though the most dramatic increase in alpha diversity occurred in the first 6 years of life [30]. Consistent with the gradual increase in alpha diversity seen in this tri-national study, Canadian (n = 42 per group) and Italian (n = ∼20 per group) studies did not show any significant difference in alpha diversity between elderly and young adult or mid-aged participants [28, 68]. Although a cohort of 3653 subjects age 18–87 from the United States had a significant positive association between age and alpha diversity, a cohort of 907 American men age 78–98 failed to reach a similar significant association, though alpha diversity was consistently (but non-significantly) associated with age in all participants with higher health metrics [31]. These findings suggest that although alpha diversity increases over the course of the human lifespan, significant differences in alpha diversity may or may not appear depending on the cohort and the age difference between the groups being compared. It is also worth noting that none of these human studies report a significant decrease in alpha diversity with age, apart from adverse health conditions or extreme age (90+ years).
Although frailty does not accompany age at the same rate across humans, frailty correlates nearly perfectly with age in mice housed in standardized laboratory conditions [69]. A compilation of bacterial diversity data in publicly available gut microbiome data sets with aged and young laboratory mice revealed that in seven of the eight data sets evaluated, at least one alpha diversity index was significantly higher in the aged mice compared to the young [70]. Three additional mouse studies and one rat study showed either higher alpha diversity in aged versus young animals or increasing alpha diversity into old age in these model organisms [71-74]. However, it should be noted that another mouse study found significantly reduced alpha diversity in aged mice compared to young [75]. Nonetheless, the consensus in the literature is that gut alpha diversity increases over the lifetime of humans and rodent models, apart from disease and microbiome-altering stressors.
3.2 Taxa Affected by Aging
The phyla generally exhibiting change with age are Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Verrucomicrobia. Additionally, the ratio of the relative abundance of Firmicutes and Bacteroidetes has been cited as an important metric in health and aging. However, our observation here is that although some studies show a moderate increase in the Firmicutes:Bacteroidetes ratio with age up until around age 70, increases in this ratio are generally nonsignificant when compared to younger adults in human studies [26, 28, 68], and its usefulness as a metric of aging is not supported by the data in mouse studies [70, 76, 77]. In humans, phylum Firmicutes tends to increase with age but then decrease after a certain point, often around age 70 [26, 27, 29, 78], whereas in mice, Firmicutes has a consistent pattern of increasing rather than decreasing with age [75, 76, 79]. It should be noted, however, that not all mouse studies consider extreme age as some of the human studies do.
Lower taxa in phylum Firmicutes that change with age are listed in Table 3. Notable taxa from this table are SCFA producers, particularly F. prausnitzii. F. prausnitzii is a member of family Ruminococcaceae (known as Clostridium cluster IV in many of the sources on aging cited here) and decreases with age in mice and in a variety of human populations [29, 32, 59, 72, 75, 78, 80, 81]. F. prausnitzii appears to be an important gut commensal for health, as the loss of this species has been associated with several bowel diseases [82]. Although F. prausnitzii tends to decrease with aging, family Ruminococcaceae as a whole tends to increase with age in both mice and humans [27, 68, 69, 73, 74]. In contrast, family Lachnospiraceae (known as Clostridium cluster XIVa in many of the aging sources cited here) shows opposite trends with aging in humans and murine models. There is agreement in the literature that family Lachnospiraceae as a whole decreases with age in humans and rats [29, 74, 80] and that distinct genera within Lachnospiraceae also decrease with age in humans and rats [29, 78, 80]. However, the literature on mice shows increases in Lachnospiraceae with age [69, 73, 75], demonstrating that caution should be used when interpreting the results of animal studies as mouse gut microbiome data is not always directly applicable to human medicine.
| Taxon | Direction | Details | Reference |
|---|---|---|---|
| Firmicutes:Bacteroidetes ratio | Up | Italian elderly (age 63–76, n = 22) had higher F:B ratios compared to younger adults (age 25–40, n = 20) | [28] |
| Up | 1550 healthy Ukrainian subjects showed the average F:B ratio increasing with each decade up to age 69 | [26] | |
| Down | Ukrainian and Canadian adults over age 70 had decreased F:B ratios compared to middle-aged adults | [26, 68] | |
| No change | A meta-analysis of eight mouse data sets did not find a significant difference in the F:B ratio in aged vs. young mice | [70] | |
| P. Firmicutes | Up then down | Firmicutes:
|
[26, 27, 29, 78] |
| Up | Significantly increased Firmicutes in age 70+ compared to age 30–50 (mid-aged) Canadian subjects (n = 42 per group) | [68] | |
| Up | Increased Firmicutes with age in laboratory mice | [75, 76, 79] | |
|
P. Firmicutes C. Clostridia O. Clostridiales |
Up | Increased Clostridiales with age in mice and rats | [72–74, 76] |
|
P. Firmicutes C. Clostridia O. Clostridiales F. Ruminococcaceae |
Up | Increased family Ruminococcaceae in Irish elderly compared to aggregate young adult data from several other studies | [27] |
| Up | Increased family Ruminococcaceae with age in mice and rats | [69, 73, 74] | |
| Down | Decreased Faecalibacterium prausnitzii with age in mice and in a variety of human populations | [29, 32, 59, 72, 75, 78, 80, 81] | |
| Up | Increased F. prausnitzii in Irish elderly compared to aggregate young adult data from several other studies | [27] | |
|
P. Firmicutes C. Clostridia O. Clostridiales F. Lachnospiraceae |
Down | Decreased family Lachnospiraceae with age in humans and rats | [27, 29, 74, 80] |
| Up | Increased family Lachnospiraceae with age in mice | [69, 73, 75] | |
| Down | Decreased genus Roseburia elderly compared to young adult:
|
[29, 75, 78, 80] | |
| Down | Decreased genus Coprococcus elderly compared to young adult:
|
[29, 80] | |
| Down | Decreased genus Dorea in elderly compared to young adult Finnish subjects | [78] | |
| P. Bacteroidetes | Down then up | Bacteroidetes troughed in healthy Japanese and Ukrainian subjects around age 60 but increased after age 70 | [26, 29] |
| Up | Bacteroidetes:
|
[27, 76, 78, 79] | |
|
P. Bacteroidetes C. Bacteroidia O. Bacteroidales |
Up | Genus Bacteroides was:
|
[27, 29, 68, 70, 83] |
| Up | Genus Alistipes was:
|
[27, 32, 69, 70] | |
| Up | Genus Parabacteroides:
|
[27, 70] | |
| Up | Increased family Porphyromonadaceae with age in:
|
[29, 59, 69] | |
| P. Actinobacteria | Down | Decreased Actinobacteria:
|
[27, 29] |
| Up | Increased Actinobacteria with age in Ukrainian subjects | [26] | |
|
P. Actinobacteria C. Actinobacteria O. Bifidobacteriales F. Bifidobacteriaceae |
Down | Decreased genus Bifidobacterium:
|
[28, 29, 80, 81, 83] |
| P. Proteobacteria | Up | Increased Proteobacteria:
|
[29, 68, 78, 79] |
| Down | Decreased Proteobacteria in elderly compared to young adult Irish subjects | [27] | |
|
P. Proteobacteria C. Gammaproteobacteria |
Up | Increased family Enterobacteriaceae and genera Enterobacter and Escherichia in elderly compared to young or mid-aged adult:
|
[29, 59, 68, 81, 83] |
| P. Verrucomicrobia | Up | Increased Verrucomicrobia in age 70+ Canadian subjects compared to mid-aged controls | [68] |
| Down | Decreased Verrucomicrobia with age in a meta-analysis of eight mouse data sets | [70] | |
| Up | Genus Akkermansia was:
|
[27, 32, 68, 80] | |
| Down | Decreased genus Akkermansia with age in mice and rats | [69-71, 74-76] |
Opposite to Firmicutes, Bacteroidetes initially decrease with age but increase again starting around age 70 [26, 27, 29, 76, 78, 79]. Specific changes are listed in Table 3. More than 50% of the Bacteroidetes reads in a study of age 65+ Irish subjects (n = 161 aged and 9 young controls) were from the genera Bacteroides, Alistipes, and Parabacteroides, whereas these three genera comprised no more than 27% of the Bacteroidetes reads in any of the young controls [27]. Furthermore, there were several species in these three genera that were not found in the young controls, and all three genera were negatively correlated with SCFA production in mice [70]. This finding is corroborated in other aging studies finding Alistipes was higher with age in mice and a large human cohort [32, 69], and Bacteroides higher in Japanese and Canadian elderly subjects [29, 68, 83]. However, Alistipes and Parabacteroides do not have purely detrimental effects on aging, as one study of 201 elderly subjects found Alistipes and Parabacteroides to be negatively associated with visceral adipose tissue, a risk factor for cardiovascular problems and metabolic syndrome [44]. In addition to increases in these three genera implicated in inflammation, the beneficial family Porphyromonadaceae [44, 45] increases with age in mice and humans [29, 59, 69], further illustrating how the general increase in minor phyla with age is not necessarily detrimental.
As in ovarian cancer, Actinobacteria at the phylum level does not change in definitive directions with age [26, 27, 29]. There is, however, a strong consensus that genus Bifidobacterium and its associated benefits decrease with age. The decrease in Bifidobacterium is among the most documented changes in the gut microbiome with age, as its decrease was detected in Japanese, German, French, Swedish, and two separate Italian cohorts (n = 30–40 elderly and 15–25 young adults per cohort) [28, 29, 80, 81, 83]. The increased inflammation seen in aging may be due in part to decreased SCFAs from Bifidobacterium and the Firmicutes families Ruminococcaceae and Lachnospiraceae. It is interesting to note that Bifidobacterium was overrepresented in a small cohort of healthy Italian centenarians and supercentenarians [80]. Proteobacteria tends to increase with age at the phylum level [29, 68, 78, 79]. Additionally, increases in genera Enterobacter and Escherichia with age have been documented in Japanese, Indian, Canadian, German, Italian, French, and Swedish populations [29, 59, 68, 81, 83]. Enterobacter and Escherichia are opportunistic pathogens known to cause inflammation, gut dysbiosis, and enteric and urinary tract infections [84], making these genera among the more detrimental gut microbiome changes associated with aging.
Although Verrucomicrobia was increased in age 70+ Canadian subjects compared to mid-aged controls, this phylum was significantly decreased with age in the meta-analysis of eight mouse data sets [68, 70]. Likewise, Akkermansia, the dominant Verrucomicrobia genus in the gut, was increased in three elderly cohorts compared to younger adults and positively associated with age in another large cohort [27, 32, 68, 80]. However, the overwhelming consensus in rodent studies is that Akkermansia decreases with age in mice and rats [69–71, 74–76]. As with family Lachnospiraceae, this discrepancy between the normal aging pattern of gut Akkermansia in these human studies compared to the rodent studies is a reminder that although rodent model gut microbiota reasonably mimic those of the human gut, not all experimental results are directly applicable to human medicine.
3.3 Unhealthy Aging
There are a few noteworthy changes in the gut microbiota in unhealthy aging when compared with generally healthy, normal aging. Compared to its general increase with age, phylum Firmicutes has decreased in patients with Alzheimer's disease compared to healthy controls and decreased in long-stay care residents compared to community-dwelling elderly [33, 85], perhaps due to an acceleration of the decrease in Firmicutes that occurs in extreme age [26, 29, 86]. Despite being higher in the generally healthy elderly compared to younger adults, Ruminococcaceae (Clostridium cluster IV) abundance and diversity were shown to be lower in institutionalized elderly compared to healthy young controls [67, 68, 78], though higher in critically ill older patients leaving the intensive care unit dead (n = 23) compared to those released alive (n = 49) [87].
The remaining noteworthy taxa show trends in unhealthy aging that are consistent with their trends in normal aging. In phylum Firmicutes, the member of family Lachnospiraceae (Clostridium cluster XIVa), particularly Roseburia and Coprococcus, were decreased in long-stay care residents compared to community-dwelling elderly [33]. In phylum Bacteroidetes, genus Bacteroides diversity was decreased, whereas Bacteroides abundance was increased in institutionalized elderly compared to young adult subjects [67], and Bacteroides abundance was positively associated with mortality in elderly men (n = 907) [31]. Parabacteroides abundance was increased in long-stay care residents compared to community dwelling elderly, and Alistipes was a dominant taxon in a co-abundance group corresponding to this long-stay group [33]. Finally, consistent with its general decrease with age, Bifidobacterium from phylum Actinobacteria was decreased in institutionalized elderly compared to healthy young controls [67].
4 Crossroads of Aging and Ovarian Cancer
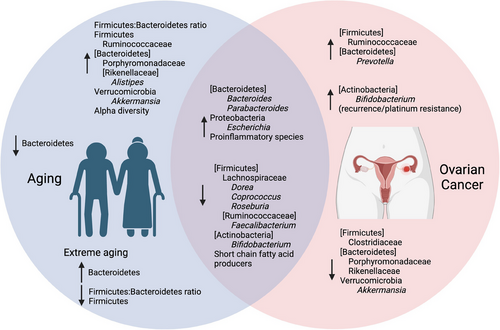
The contrasts and commonalities of gut microbiome changes in ovarian cancer and aging are summarized in Figure 3. The reports reviewed indicate that, although alpha diversity increases with age but decreases in some cases of pathological aging, there are no differences in alpha diversities with respect to ovarian cancer presence or stage, though a prolonged decrease in alpha diversity during platinum chemotherapy may be prognostic of a poor response to treatment. In all these comparisons, ovarian cancer patients and non-ovarian-cancer-bearing control subjects were matched with respect to age and several other demographic variables, such as geography, menopause status, and body mass index. Thus, it appears that ovarian cancer does not significantly affect gut microbiome alpha diversity above the general effects of aging. Several genera from phylum Firmicutes decreased with both age and ovarian cancer. Lower abundances of family Lachnospiraceae (Clostridium cluster XIVa) are found in aged compared to younger subjects [29, 74, 75, 78, 80], in ovarian cancer patients compared to healthy controls [17, 18], in ovarian cancer patients with late compared to early stage disease at diagnosis [17], and in ovarian cancer patients with recurrence compared to no recurrence in a 2-year timeframe [19]. Likewise, the Actinobacteria genus Bifidobacterium is decreased both with age [29, 80, 81, 83] and in ovarian cancer patients compared to healthy controls [18] but has been shown to be increased in cases of ovarian cancer complications such as recurrence and lymph node metastasis [18, 24] Family Ruminococcaceae (Clostridium cluster IV) exhibits mixed trends in ovarian cancer, namely, some members being increased with late-stage ovarian cancer [19], whereas some members are decreased with ovarian cancer or negatively associated with platinum resistance [17, 18]. Similar to their mixed trends in ovarian cancer, members of family Ruminococcaceae tend to increase with normal aging but may decrease with frailty [27, 68, 78, 80]. Thus, it is difficult to make connections between aging and ovarian cancer with respect to family Ruminococcaceae as a whole. One connection that can be made, however, is that genus Faecalibacterium appears to decrease in both healthy aged compared to healthy young subjects and in ovarian cancer patients compared to healthy subjects (though not necessarily compared to subjects with benign gynecological conditions) [16, 17, 29, 32, 59, 72, 75, 78, 80, 81]. Meanwhile, phylum Proteobacteria is increased with ovarian cancer presence and severity [16, 18, 24] as well as with age [29, 68, 78, 79].
4.1 Discussion of Shared Changes Between Aging and Ovarian Cancer
The decrease in Lachnospiraceae members, Faecalibacterium, and Bifidobacterium with an increase in Proteobacteria exemplifies situations in which ovarian cancer appears to exacerbate unfavorable changes already taking place in the gut microbiome during the aging process. Bacteria from Lachnospiraceae and Ruminococcaceae are SCFA producers, which digest dietary fiber into primarily acetate, propionate, and butyrate. Through the action of their receptors, SCFAs promote gut barrier integrity and reduce inflammation and oxidative stress, and improve immunity, metabolism, and weight management [41]. Faecalibacterium utilizes acetate and produces butyrate, giving this genus a symbiotic relationship with other SFCA producers, and its abundance can discriminate patients with intestinal disease from healthy control subjects [82, 88]. Bifidobacterium, though associated with platinum resistance due to its lactate production [18], is also an acetate producer, promotes the growth of propionate and butyrate producers, and is itself promoted by butyrate, forming a symbiotic relationship with the other SCFA producers in the gut [41]. Proteobacteria have been associated with metabolic disorders, inflammatory bowel disease, and several other inflammatory pathologies [49], though one study suggests that the shrinking of the core gut microbiota with age, which is accompanied by an increase in the relative abundance of minor phyla such as Proteobacteria, may, in healthy aging, also accommodate an increase in health-related species [80]. Age-associated decreases in species that reduce metabolic imbalance and inflammation with increases in species associated with these pathologies may be one of the factors that predispose older women to ovarian cancer, whereas the further decrease in beneficial bacteria and increase in proinflammatory bacteria with ovarian cancer could be either causal or symptomatic of the disease.
4.2 Discussion of Contrasting Changes Between Aging and Ovarian Cancer
Other taxa exhibit opposite trends in ovarian cancer compared to aging. The Bacteroidetes family Porphyromonadaceae is increased in aged compared to younger subjects [29, 59, 69] but decreased in ovarian cancer patients compared to healthy controls [18]. Genus Akkermansia from phylum Verrucomicrobia was shown to be decreased in ovarian cancer patients compared to patients with benign disease in one report [16] but increased with age in all the reports, noting differences in this genus with age in healthy human subjects [28, 32, 68, 80]. The change in direction of Porphyromonadaceae and Akkermansia with ovarian cancer compared to the general trend of aging may be examples of ovarian cancer opposing normal and healthy changes in the gut microbiome with age or taking advantage of pathological dysbiosis. Bacteria from family Porphyromonadaceae have been associated with reduced obesity, a risk factor for ovarian cancer, and reduced visceral adipose tissue, which is a preferred metastatic site [44, 89]. A. muciniphila is not only negatively correlated with obesity, but also feeds on colonic mucus and, like Bifidobacterium species, produces acetate, which promotes the growth of other beneficial SCFA producers [41, 90]. The detrimental nature of reduced Porphyromonadaceae and Akkermansia in ovarian cancer is further supported by studies that show lower levels of species from these taxa in unhealthy elderly compared to younger adult control subjects [78, 87, 91].
4.3 Gut Microbiome Interventions Tailored to the Aging Ovarian Cancer Patient
The first means of improvement of the gut microbiome in any situation is to increase consumption of fresh vegetables, as these contain large amounts of dietary fiber that feed desirable gut commensals [32, 92–95]. There is also evidence that exercise can have desirable effects on the gut microbiome [96] though the impracticality of prescribing exercise to older women are already suffering from ovarian cancer may render exercise better suited to the realm of prevention through lifelong habits. Prebiotic and probiotic supplementation is an additional means that can be used to improve the composition of the gut microbiome [79, 94]. Supplementation may be more conducive to patient compliance as it does not require lifestyle changes, though it is most useful in concurrence with improvements in diet and exercise.
Supplementation specific to species reduced in ovarian cancer and aging has not been extensively explored but may prove to be of value, but more research is needed to explore how selectively promoting one type of bacteria would affect the general balance of the gut microbiome. A variety of over-the-counter prebiotics are available, with ingredients, such as inulin, xylo-oligosaccharides, and digestion-resistant starch to feed common SCFA producers. Pectin is being explored as a specific prebiotic for Faecalibacterium [82], whereas berberine has been shown to indirectly support Akkermansia by stimulating host intestinal mucus secretion [97]. Galacto-oligosaccharides and inulin have been shown to promote Porphyromonadaceae species [98, 99].
A digestion resistant starch probiotic has been shown to increase Bifidobacterium in older subjects [68], though supporting Bifidobacterium growth may be better as a preventative measure for healthy aging rather than for diagnosed ovarian cancer patients undergoing chemotherapy given the apparent role of Bifidobacterium in platinum resistance [18]. A possible tactic for combatting excess lactate in chemotherapy resistance is to selectively support the growth of lactate utilizing bacteria, particularly from the Veillonellaceae family, as observed in the Italian chemotherapy study, but there is limited research on supporting this family as several of its members are considered pathobionts or causes of gastrointestinal discomfort, especially in children [100, 101]. An additional contributor to the lack of research on gut microbiome interventions supporting lactate utilizers is that lactate is generally considered a beneficial bacterial metabolite, along with propionate and butyrate. However, there is evidence that lactate accumulation in the colon is a sign of gut dysbiosis in adults and that a colonic pH of 6.5 (compared to the relatively acid pH of 5.5 found in some intestinal disorders) promotes a healthy quorum of lactate utilizing bacteria that keep gut lactate levels in check [102]. Mechanisms of maintaining a more basic colonic pH involve dietary modulation (less starch and more fiber) and lactate utilizing bacteria probiotics, bringing us back to the initial problem of a lack of research on selectively increasing lactate utilizing bacteria in the gut.
Traditional Chinese medicines have been shown to modulate the gut microbiome and reduce tumor burden in rodent models of ovarian cancer. However, the changes seen in the gut microbiome in each study do not overlap well between studies, do not line up well with changes seen in human ovarian cancer patients compared to control subjects, and do not result in the experimentally treated animals having a gut microbiome that better resembles their respective tumor-naïve controls [23, 34, 103]. The lack of similarity in gut microbiome changes between experiments is reasonable considering that each study was testing a different compound. However, the dissimilarity to the major changes seen in human ovarian cancer patients and the distinct profile in the treated animals compared to tumor-naïve controls indicate that these compounds promote a unique gut microbiome composition rather than returning the composition to a pre-cancer state. More work is needed to establish whether and how the gut microbiome modulation caused the improvement in tumor burden in these studies.
Finally, FMT may be a viable option for modulating the gut microbiome in ovarian cancer. There is evidence that FMT can modulate ovarian cancer outcomes in mice, and FMT would remodel the whole gut microbiome, as is sometimes necessary to realize the benefits of gut microbiome modulation [16, 25]. FMT has been used as an aid to immunotherapy for other types of cancer [104], though its use remains controversial due to the possibility of infection due to the addition of foreign microbiota [105]. One consideration that may lessen this undesired effect of FMT is to use the patient's own feces banked prior to ovarian cancer treatment. Although the gut microbiome at the time of diagnosis differs from that of healthy individuals [17, 18], it is in better condition at that point than after platinum chemotherapy, and using a stool sample derived from the patient reduces the risk of introducing foreign pathogens. As methods for FMT quality control improve, additional benefits of FMT from healthy donors in the treatment of ovarian cancer may become apparent. It also remains to be seen whether FMT from healthy young donors to treat older ovarian cancer patients could help with age-related gut dysbiosis or whether older patients would benefit more from transplantation of a healthy aged-type gut microbiome such as those seen in extreme longevity.
5 Gaps in the Literature
The current evidence for causal relationships between gut microbiome changes and ovarian cancer symptoms is limited to the two studies in which treatment of ovarian cancer-bearing mice with FMT from ovarian cancer patients promoted tumor growth via IFN-γ and CD8+ T cell or Hedgehog and TNF-α/IL-1β pathways [16, 17]. Research on the benefits of gut microbiome remediation in ovarian cancer treatment is limited to a few studies in which mice have benefitted from FMT from healthy donors or their own stool banked prior to chemotherapy [16, 17, 25], or pilot studies showing gut microbiome modulation via Traditional Chinese Medicine [23, 34, 103]. Importantly, to our knowledge, there are no clinical trials on gut microbiome remediation to enhance ovarian cancer treatment. More research is needed to establish whether the changes in the gut microbial signature with ovarian cancer are a driver of ovarian cancer or a mere consequence, and whether gut microbiome remediation is a useful supplement to ovarian cancer treatment. Furthermore, although there is decent literature coverage on the gut microbiome in cancer, most reports cover colorectal cancer and few cover ovarian cancer specifically, meaning that there is limited data on microbiota signatures that could aid in ovarian cancer diagnosis. Of these reports, even fewer cover all three topics of gut microbiome, aging, and cancer. To our knowledge, none of those that consider all three topics look at ovarian cancer specifically [106-108].
Another aspect of gut microbiota, aging, and ovarian cancer that warrants attention is how age and menopausal status affect the gut microbiota signature in ovarian cancer. A recent analysis of a multi-cohort gut microbiome data set was able to identify gut microbiota signatures of five diseases, including colorectal cancer, that were masked until stratifying subjects by age into three groups, young (20–39 years), middle-aged (40–59 years), and elderly (60+ years) [95]. The ovarian cancer patients in articles reviewed here were generally aged between 40 and 70 years, but none of the reports differentiated patients by age. As in the case of colorectal cancer and other diseases, such stratification may reveal signatures that are not apparent across the entire spectrum of ages at ovarian cancer diagnosis. Additionally, because the gut microbiota have notable influence on estrogen reabsorption, especially when there is low estrogen of ovarian origin [35, 38], further studies considering aging and menopausal status on estrogen metabolism by gut microbiota need to be conducted. Finally, it remains to be seen how the decrease in gut barrier integrity with age may affect the microbiome of the ovaries and the intraperitoneal tumor microenvironment. It is known that the ovaries and upper reproductive tract are colonized by bacteria and that ovarian microbial signatures differ between patients with ovarian cancer and patients with benign gynecological conditions [19, 109]. The origin of microbiota in the upper reproductive tract is currently presumed to be due to migration from the lower reproductive tract. The lower reproductive tract microbiome is heavily influenced by the gut microbiome due to the proximity of the vagina to the anus, but how direct bacterial leakage from the gut into the peritoneal cavity may affect the ovarian microbiome, particularly with age, is understudied. Based on the literature reviewed here, we believe that decreased gut barrier integrity could influence the ovarian microbiome through both direct migration of bacteria and modulation of the peritoneal immune response [25, 50, 51].
6 Conclusions and Future Directions
Although there are a few published reports featuring data on the gut microbiome in ovarian cancer patients and animal models, as well as a larger body of literature on how the gut microbiome changes with age, there is a paucity of literature considering the gut microbiome, aging, and ovarian cancer. A review of the literature on the gut microbiome in ovarian cancer or aging shows common trends, including a decrease in bacteria that produce SCFAs with an increase in bacteria implicated in obesity and metabolic disease with either condition. Moreover, ovarian cancer patients show a further decrease in obesity-reducing bacteria compared to age-matched control subjects and against the trend in normal, healthy aging. Improvements in research coverage of the effects of aging on the gut microbiome in ovarian cancer could lead to improvements in both ovarian cancer diagnosis and treatment by providing more comprehensive, age-stratified data sets of changes in the gut microbiome with ovarian cancer, along with detailed insight and research on how to modify the aging ovarian cancer gut microbiome to improve treatment outcomes.
Author Contributions
Conceptualization: Gena M. Dominique. Writing–original draft preparation: Gena M. Dominique and Catherine Hammond. Writing–review and editing: Gena M. Dominique, Catherine Hammond, and M. Sharon. Stack Visualization: Gena M. Dominique and M. Sharon Stack. Supervision: Gena M. Dominique. Investigation: Gena M. Dominique and Catherine Hammond. Project administration: Gena M. Dominique. Funding acquisition: M. Sharon Stack and Gena M. Dominique All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank Nathaniel Dominique for technical support with reference management software. All figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The authors have nothing to report.




