Skeletal muscle and childhood cancer: Where are we now and where we go from here
Abstract
Skeletal muscle (muscle) is essential for physical health and for metabolic integrity, with sarcopenia (progressive muscle mass loss and weakness), a precursor of aging and chronic disease. Loss of lean mass and muscle quality (force generation per unit of muscle) in the general population are associated with fatigue, weakness, and slowed walking speed, eventually interfering with the ability to maintain physical independence, and impacting participation in social roles and quality of life. Muscle mass and strength impairments are also documented during childhood cancer treatment, which often persist into adult survivorship, and contribute to an aging phenotype in this vulnerable population. Although several treatment exposures appear to confer increased risk for loss of mass and strength that persists after therapy, the pathophysiology responsible for poor muscle quantity and quality is not well understood in the childhood cancer survivor population. This is partly due to limited access to both pediatric and adult survivor muscle tissue samples, and to difficulties surrounding noninvasive investigative approaches for muscle assessment. Because muscle accounts for just under half of the body's mass and is essential for movement, metabolism, and metabolic health, understanding mechanisms of injury responsible for both initial and persistent dysfunction is important and will provide a foundation for intervention. The purpose of this review is to provide an overview of the available evidence describing associations between childhood cancer, its treatment, and muscle outcomes, identifying gaps in current knowledge.
1 INTRODUCTION
Increased survival rates for childhood cancer are a direct reflection of major therapeutic and diagnostic advances made in recent decades. Today, the expected 5-year survival rate for childhood cancer exceeds 85%.1, 2 As the number of childhood cancer survivors continues to grow, so does the number of investigations designed to identify risk factors for limitations in physical function,3-5 adverse health outcomes,6, 7 and reduced quality of life in long-term survivors.5, 8 In the general population, skeletal muscle (muscle) health is a clinical indicator of metabolic disease and fall risk. Poor muscle health is associated with impaired mobility and early mortality.9-11 In children, low muscle mass and fitness are associated with metabolic risk.12 Muscle strength in both children and adolescents is also a powerful predictor of insulin sensitivity and metabolic risk in adulthood.13 Childhood cancer patients receive aggressive chemotherapy and radiation treatment during crucial physiological development. Survivors are at risk for treatment-related musculoskeletal late effects.14 Although much attention has been directed at bone health, the acute and long-term effects of childhood cancer and treatment on muscle health have not been fully elucidated.
There are over 600 muscles in the human body, accounting for 40% of total body mass. Muscle serves a variety of health and functional purposes across the lifespan. It is the mechanistic machinery of daily movement and protects other organs against trauma. Muscle is the largest source of body protein and the main reservoir of amino acids used by other tissues for regeneration and repair. Further, it is a major site of glucose homeostasis, energy mobilization, and even has the capacity to act as a secretory organ, releasing factors into circulation with autocrine and paracrine effects. Healthy muscle is resilient; it is strong, flexible, abundant, and adaptable. Muscle quality and function are the primary measures of muscle health (Figure 1), integrating the lifetime impact of a variety of influences such as diet, exercise, and disease. Muscle fitness integrates the quantity and quality of muscle and neurological coordination to provide a measure of functional capacity, measured as strength, endurance, and power. Given that key muscle development occurs early in life, a time when childhood cancer patients are exposed to aggressive chemotherapy and radiotherapy treatments, they are at great risk for early declines in muscle health that may contribute to adverse outcomes later in life.
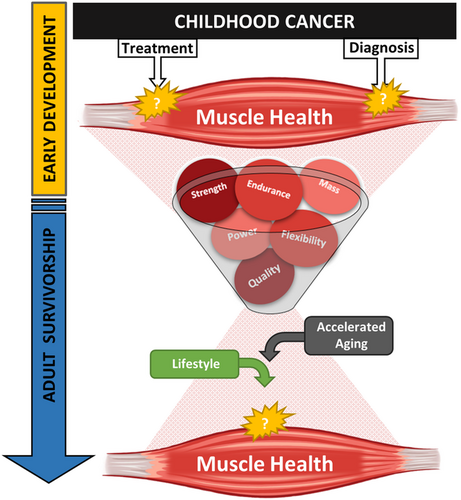
Impaired muscle quality and function are noted in both childhood cancer patients15-19 and survivors.3, 5, 17, 20-28 However, the pathophysiologic mechanisms responsible for these impairments are not well documented. This is partly due to limited research access to muscle tissue samples, and to difficulties surrounding noninvasive investigative approaches for muscle assessment. Given muscle's contribution to mobility, cardiometabolic health, and quality of life, identifying treatment and diagnostic specific risk for muscle impairments will undoubtedly improve functional outcomes for both patients and long-term survivors. The purpose of this review is to provide an overview of the available evidence describing associations between childhood cancer, its treatment, and muscle outcomes, identifying gaps in current knowledge.
2 MUSCLE ASSESSMENT METHODS
2.1 Mass
Both imaging and nonimaging techniques are used to measure muscle mass. Bioelectrical impedance analysis (BIA) is a nonimaging technique routinely used in clinical settings. Despite BIA being inexpensive, quick, and easy to perform, BIA may not provide the most accurate estimation of muscle mass in children with cancer; BIA measurements are affected by hydration status, often compromised in hospitalized children. Also, no population-specific equation for children with cancer to predict fat-free mass exists. Air displacement plethysmography (ADP) is another nonimaging method previously used to evaluate body composition in children with cancer.29, 30 Although ADP is noninvasive and time efficient, estimates of fat and fat-free mass can be affected by a patient's clothing, movement, or body temperature.31 ADP is also unable to identify regional differences in body composition, which is important when evaluating relationships between muscle mass and function to understand the whole sarcopenic phenotype in this population.
Fortunately, multiple imaging techniques are available, offering advantages over BIA and ADP. Dual-energy X-ray absorptiometry (DXA) is commonly used to assess body composition and fat-free mass in clinical settings. A DXA scan has a modest cost, short scan time, and is relatively accessible in a hospital setting. However, despite these advantages, DXA can only distinguish regional differences in body composition in two dimensions. Given that development of sarcopenia is undoubtedly complex, more detailed, three-dimensional images are better suited to divulging the pathophysiological processes underlying muscle wasting in children with cancer. Magnetic resonance (MR) and computed tomography (CT) are considered gold standard imaging techniques to assess regional changes in lean mass, specifically muscle. Both these methods can quantify change in intramuscular structure such as cross-sectional area, volume, or intramuscular adiposity.32, 33 Regional three-dimensional images at predefined anatomical landmarks can also be used to estimate whole body muscle mass.34, 35 Unfortunately, both MR and CT imaging are costly, require highly sophisticated machinery, and are resource intensive. Alternatively, ultrasound has emerged as a potential alternative for skeletal muscle imaging. Muscle ultrasound has been performed previously in hospitalized children to assess diaphragm and lower leg muscle thickness.36-40 Panoramic ultrasound is a reliable and valid tool, comparable to MRI, to quantify change in muscle structure and mass in adults.41-43 Although not yet validated in children, panoramic ultrasound may be a cost efficient, bedside capable, less invasive alternative to MR and CT techniques in children with cancer. Other ultrasound methodologies such as elastography and contrast-enhanced ultrasound can be used to assess mechanistical properties and microvascular blood flow of muscle,44-47 and are promising methods to provide insight into the pathophysiological processes underlying muscle wasting in children with cancer.
One of the major processes that underlies muscle mass homeostasis is muscle protein synthesis. Traditionally, muscle biopsies are used in combination with radioisotopes to evaluate muscle protein synthesis in adults.48, 49 However, in children access to muscle tissue is limited. Thus, there is very limited knowledge of muscle protein synthesis and whether it is altered in children with cancer. Positron-emission tomography (PET) is a functional imaging technique that is routinely used in the diagnosis and treatment of children with cancer. Sometimes, a PET and CT scan are performed simultaneously to provide information about function, size, and shape of tumors and the surrounding structures. Furthermore, PET imaging can also provide dynamic and quantifiable information of other tissues beside tumors. PET with L-[methyl-11C] methionine can be used to assess both basal and responsive muscle protein synthesis rates,50-52 and more recently has been validated as a less-invasive alternative to the traditional L-[ring 13C6] phenylalanine infusion with serial muscle biopsy in adults.53 However, the use of PET to evaluate muscle protein synthesis has not been validated in children.
2.2 Strength
A wide variety of methods are available to evaluate muscle function. Muscle strength is a component of both sarcopenia32, 54 and frailty,55 two phenotypes indicating comprised muscle health. Manual muscle testing (MMT) is readily used in clinic to determine the grade of strength in patients with neuromuscular dysfunction.56 Unfortunately, this method provides more qualitative information and relies on subjective interpretation of muscle performance. Thus, it is a less than ideal method to quantify muscle strength changes in children with cancer, especially when performed by different testers. Hand-held dynamometry (HHD) is a cost- and time-efficient method that can provide a basic quantifiable measure of strength. Strength is assessed by a “make” or “break” test by which a patient has to meet the resistance or overpower the force imparted on them by the test administrator using a hand-held dynamometer. Although this method can be used in patients as young as 2 years old,57 it can be difficult to perform when a patient has a body-size advantage over the test administer or the patient is not positioned correctly, potentially affecting the validity of the test.58, 59 Fixed and portable dynamometers are popular alternative methods in both research and clinical settings. They are easy to use, cost-effective, and provide reliable and valid measures of strength.60-65 They limit the margin of user error compared to HHD. Although these methods are well suited to both clinical and research environments, using minimal space and requiring minimal set-up time, they are unable to assess dynamic movement. As such, isokinetic dynamometry is the gold standard method to assess muscle strength. This computerized method can capture concentric, eccentric, and isotonic/isometric maximal and endurance strength of multiple muscle groups. Unfortunately, this equipment is costly and requires specialized training and substantial physical space. Alternatively, hand grip strength via HHD is used in the diagnostic criteria for aging muscle phenotypes.32, 54, 55 Not only is it easily administered and does not require advanced training, it is also portable making it well suited for use in the hospital setting. Although hand grip strength is associated with long-term health in adolescents,66 it is not a direct measure of the major locomotive muscle groups in the legs. Thus, hand grip strength may inaccurately estimate the prevalence and impact of muscle weakness in children with cancer. Overall, the interpretation of muscle strength and changes there-of should be considered in the context of the advantages and disadvantages of the assessment methods used.
3 MUSCLE HEALTH AND CHILDHOOD CANCER
3.1 Acute outcomes
Muscle weakness and low lean mass in childhood cancer patients and survivors are consistently reported (Table 1).5, 20, 22-24, 67-73 These acute outcomes likely result from a combination of factors, including the cancer itself, aggressive multimodal therapies, as well as systemic changes including inflammation, hormone concentrations, and nutritional status. Unfortunately, few studies have focused on how these factors directly affect skeletal muscle. However, in children with cancer, muscle mass changes occur independently of changes in bone mineral content, fat mass, and total body mass, suggesting that muscle can and should be considered explicitly and independently from body mass index or adiposity. Yang and Choi74 illustrated this in a propsective study for longtitudinal assessment of nutritonal status and body composition in newly diagnosed heamatologic malignancy (n = 19) and solid tumor (n = 11) patients. Among 30 children (mean age: 10.9 ± 3.8 years, 70% male), DXA showed that median fat-free mass significantly decreased from 27.4 kg (range, 11.5–53.5 kg) to 26.9 kg (14.4–50.6 kg, p = 0.008) during the first month of cancer treatment, occurring in the absence of total body mass change.
| Author (Country) | Year published | Population | Study design | N | Age | Muscle mass assessment method | Muscle function assessment method | Results |
|---|---|---|---|---|---|---|---|---|
| Children with cancer | ||||||||
|
Yang (Korea) |
2019 |
Children newly diagnosed with cancer: 70% male Controls: 73% male |
Prospective |
30 patients: 19 hematological malignancy,11 solid tumor 30 controls |
Patient mean (SD): 10.9 (3.8) years Control mean (range): 12.3 (6.3–17.2) years |
FFM mass by DXA |
Leg FFM differed between groups Patients’ median fat-free mass ↓ during treatment |
|
|
Brinksma (The Netherlands) |
2015 | Children newly diagnosed with cancer: 48.4% male | Prospective | 133 patients: 39.8% hematological malignancy, 33.1% solid tumor, 27.1% brain tumor | Median (range): 8.1 (0.1–17.7) years | FFM via BIA |
FFM was low at diagnosis, and remained low during treatment FFM was lowest in children with brain malignancies Body mass index ↑ during treatment |
|
|
Rayar (Canada) |
2013 | Children with ALL: 63% male | Prospective | 91 patients |
Mean: 6.1 years Median (range): 5.0 (1.2–17.6) years |
SMM by DXA | SMM was low at diagnosis, ↓ with treatment, and remained low 12 months after diagnosis | |
|
Ness (United States, Canada) |
2015 | Children newly diagnosed with ALL: 65.1% male, 63.3% White | Cross-sectional | 109 patients | Median (range): 10 (4–18) years |
Lower extremity isometric strength by HHD “break” test Hand grip strength by HHD Motor development by BOT2-SF |
Patients had weaker knee extensors and hands at start of treatment Patients had worse motor abilities at diagnosis than expected |
|
|
Gocha (United States) |
2003 |
Children with ALL Controls |
Prospective |
16 patients 8 controls |
Patient median (range): 9.6 (4–15) years Controls: age- and gender-matched |
Knee extension and ankle dorsiflexion strength by HHD Functional mobility by TUG |
Patients were weaker in knee extensor and ankle dorsiflexor muscles, and had lower functional mobility than controls before start of treatment Patients’ ankle weakness worsened over 28 days of treatment Knee extensor strength was negatively correlated with functional mobility in patients |
|
|
Akyay (Turkey) |
2014 |
Children with ALL, on-therapy: 60% male Children with ALL, off therapy: 55.6% male On-therapy controls: 60% male Off-therapy controls: 55.6% male |
Prospective |
15 on-therapy patients 18 off-therapy patients 15 on-therapy controls 18 off-therapy controls |
Children on-therapy mean (SD): 9.7 (4.5) years Children off-therapy mean (SD): 12.5 (5.2) years On-therapy control mean (SD): 9.7 (4.5) years Off-therapy control mean (SD): 12.5 (5.2) years |
Hand grip strength by HHD Functional mobility by TUG |
On-therapy patients: Strength and functional mobility ↓ during treatment and was lower than off-therapy patients and controls Off-therapy patients had lower functional mobility than controls |
|
|
Schoenmaker (The Netherlands) |
2006 | Children with cancer: 50% male | Prospective | 18 patients: 17 ALL, 1 T-NHL | Range: 0–18 years | Upper and lower extremity strength by MMT and HHD |
Muscle weakness and mobility issues were most severe in the first 2 months of treatment All patients had muscle weakness in at least one muscle measured by MMT Lower extremity muscle weakness measured by HHD persisted 6 months after therapy in patients older than 5.5 years |
|
| Survivors of childhood cancer | ||||||||
|
Tonorezos (United States) |
2013 | Survivors of childhood ALL: 44.4% male, 72% White | Cross-sectional | 117 survivors | Median (range): 23 (18–37) years | Lean mass by DXA | Survivors who received cranial radiation had less lean body mass than survivors without cranial radiation | |
|
Boland (United States) |
2016 |
Survivors of childhood ALL: 47.7% male, 87% White Community controls: 47.7% male, 87% White |
Cross-sectional |
365 survivors 365 controls |
Survivor median (range): 28.5 (18.4–44.6) years Controls: 5-year age matched |
Relative lean muscle mass by DXA | Survivors had lower absolute and relative lean mass than controls | |
|
Ness (United States) |
2012 | Survivors of childhood ALL: 48.9% male, 93.7% White | Cross-sectional | 415 survivors | Median (range): 35.6 (21.9–52.3) years |
Lower extremity muscle strength by isokinetic dynamometry Functional mobility by TUG |
Survivors had dorsiflexion weakness (16.9%), plantarflexion weakness (24.6%), knee extensor weakness (30.1%), and limited functional mobility (3.6%) Knee extensor weakness increased risk for limited physical performance |
|
|
Hovi (Finland) |
1993 |
Female survivors of childhood ALL Controls |
Cross-sectional |
43 survivors 37 controls |
Survivor mean (range): 19 (14–30) years Control mean (range): 19.4 (14–30 ) years |
Elbow, knee, and hand grip isometric strength by custom-made dynamometer chair Abdominal muscular endurance by maximal sit-up test Upper body muscular endurance by maximal push-up test |
Survivors had less abdominal and upper body endurance and weaker elbow and knee extensors than controls |
|
|
Ness (United States) |
2015 |
Survivors of childhood ALL: 47.7% male, 12.1% Black Community controls: 47.7% male, 12.1% Black |
Cross-sectional |
365 survivors 365 controls |
Survivor mean (SD): 28.6 (5.9) years Control mean (SD): 28.9 (7.5) years |
% FFM and relative lean mass by DXA |
Hand grip strength by HHD Knee extensor and ankle dorsiflexion strength by isokinetic dynamometry |
Compared to controls: Female survivors with a history of cranial radiation had lower % FFM and relative lean mass (g/m2) Male survivors had lower % FFM Survivors were weaker and had less endurance in their quadriceps muscles Survivors with a history of cranial radiation had hand grip weakness |
|
Van Brussel (The Netherlands) |
2006 | Survivors of childhood ALL: 46.2% male | Cross-sectional | 13 survivors | Mean (SD): 15.5 (5.8) years |
Hand grip strength by HHD “make” test Upper and lower extremity strength by HHD “break” test |
Survivors had knee extensor weakness | |
|
Ness (United States) |
2019 |
Survivors of childhood cancer: 51.1% male, 84.1% non-Hispanic White Community controls: 48.8% male, 90.2% non-Hispanic White |
Cross-sectional |
1260 survivors 285 controls |
Survivor mean (SD): 36.4.6 (9.2) years | % lean mass and relative lean mass by DXA | Isokinetic quadriceps strength by isokinetic dynamometry |
Survivors had lower % lean mass and lower relative lean mass z-scores that controls Cranial radiation exposure >20 Gy was associated with quadriceps weakness Quadriceps weakness (–1 SD) increased risk for exercise intolerance (OR, 1.49; 95% CI, 1.23–1.82) |
- Abbreviations: ↑, increase; ↓, decrease; ALL, acute lymphoblastic leukemia; T-NHL, T-cell non-Hodgkin lymphoma; BIA, bioelectrical impedance analysis; BOT2-SF, Bruininks–Oseretsky Test of Motor Proficiency Version 2 Short Form; DXA, dual-energy X-ray absorptiometry; FFM, fat-free mass; HHD, hand-held dynamometer; MMT, manual muscle testing; PEDI, pediatric evaluation of disability inventory; ROM, range of motion; SMM, skeletal muscle mass; TUG, Time Up and Go; OR, odds ratio; CI, confidence interval
In children with cancer, both the disease and associated treatments place increased energy-substrate demands on the body to fight infection and fatigue, beyond what is normally required for normal growth and development. Further, children often experience treatment-induced nausea and vomiting. Although both over- and undernutrition in the childhood cancer population are reported,75-77 there is limited information on associations between nutritional status and body composition. There is little to no evidence that suggests nutritional status or dietary protein intake is a mediator of muscle wasting in children with cancer. Brinksma et al.78 prospectively observed significant increases in fat mass despite low energy intake during the first year following diagnosis. In this study, a total of 115 Dutch children (median age: 8.1 [range, 0.1–17.7] years, 47.0% male) recently diagnosed with cancer (41% hematological, 33% solid tumor, and 26% brain) participated in assessments of dietary energy and protein intake during various treatment protocols. Dietary intake was collected using 3-day (consecutive) food records (included 1 weekend day) within the first 1–3 weeks following diagnosis, and was repeated at 3, 6, and 12 months postdiagnosis, in between chemotherapy treatments. Food records were then transferred to a computer calculation software to quantify intakes by standard measurement units (i.e., mg and IU). To standardize for each child's specific nutritional needs, individual energy requirement (kcal/day) and individual protein requirement (g/day) were calculated and adjusted for body weight.79 Daily intakes were compared to the recommended daily allowance,80 intakes of healthy children in the Netherlands,81, 82 and calculated individual requirements. Mean energy intake (kcal) in patients was significantly lower than both recommended daily allowance and intakes of healthy children regardless of timepoint; mean percent of individual energy requirement throughout the study period was 105%. Even though overall intake was lower than required among patients, at all assessments, percent individual protein requirement (145%, p < 0.05) and recommended daily allowance of protein (181%, p < 0.01) intake were more than adequate. However, percent individual protein intake was not associated with preservation of lean mass, indicating an underlying desensitization or resistance to the nutrition-stimulated anabolism necessary for muscle mass homeostasis.83 Some patients also have lowered resting energy expenditure suggesting decreased metabolic activity.84, 85 Given that muscle has high metabolic activity,86 decreased resting energy expenditure supports the observed losses in lean mass during treatment and further underlines an insensitivity to dietary protein known to stimulate the anabolic growth process in muscle.87
Muscle wasting may vary by diagnosis, suggesting a direct effect of different malignancies. Brinksma et al.88 noted lower fat-free mass in patients with brain malignancies than patients with hematological or solid malignancies. This may be due to differences in tumor-derived cytokines89 or inflammation90-93 altering the muscle's microenvironment.94, 95 Further, the decline in muscle mass that occurs acutely has also been associated with the duration of hospitalization during induction therapy. Rayar et al.96 recently examined the patterns of change in appendicular muscle mass throughout therapy in 91 children who were less than 17 years of age at diagnosis with acute lymphoblastic leukemia (ALL). Patients received DXA scans at five separate time points during treatment with change in mass converted to Z-scores. In this study, mean muscle mass loss at diagnosis was small (–0.18 SD), and substantially increased within 6 months (–1.08 SD). This increase corresponds with the completion of remission induction therapy, which includes high-dose glucocorticoid administration. Further, the children did not fully regain their muscle mass within 12 months of diagnoses (–0.5 SD). In an evaluation of the relationship between change in muscle mass and the burden of illness (days of hospitalization), there was a significant association between mass loss at 6 months postdiagnosis and the number of inpatient days during induction therapy (r = 0.31; p < 0.05).
Muscle mass contributes to functional capacity and is implicated in body compositional changes that occur with treatment.23, 30, 97-99 Lower extremity strength is required for safe ambulation, balance, and many daily functional activities such as sitting and standing from a chair, climbing stairs, as well as maintenance of independence in daily living. There are a limited number of studies to date that have focused on muscle quality and function in patients, most of which are in children diagnosed with ALL. The vast majority of the literature has evaluated muscle strength.15-17, 22, 67
Children newly diagnosed with ALL present with muscle weakness that can worsen as treatment progresses, and may never fully recover.15-17, 67 We found strength loss in the lower extremities in 109 children (median age: 10 [range, 4–18] years, 65.1% male, 63.3% White) newly diagnosed with ALL enrolled on a physical activity trial.17 Participants were assessed for skeletal, neuromuscular, and fitness impairments within 7–10 days of initiation of chemotherapy. HHD was used to administer a “break test” for knee extension (proximal lower extremity) strength. On average, knee extensor strength was 34.2 Newtons less than expected (p < 0.01). Gocha et al.16 reported similar findings in eight children with ALL (median age: 9.6 [range, 3–15] years), assessed for strength and functional mobility before (day 0, baseline) and following delayed intensification therapy (day 28). HHD was used to measure knee extension and ankle dorsiflexion strength, and the Timed Up and Go (TUG) test was used to assess functional mobility. The TUG captures the time needed to stand from a seated position, walk 3 m, turn around, return, and sit back down in the chair.100 In this study, ALL patients on average had significantly lower knee and ankle strength, and higher TUG times (indicating impaired function) than age-matched controls. Patients with greater strength performed the TUG task faster. In this study, weakness in ALL patients progressed with delayed intensification therapy, with significant dorsiflexion strength loss (baseline 0.23 ± 0.09 kg vs. therapy 0.15 ± 0.06 kg) noted within 4 weeks. Akyay et al.15 studied 15 newly diagnosed ALL patients and 18 off-therapy patients, each with respective control-group counterparts. Hand grip strength was measured with dynamometry and functional mobility with the TUG test. Children with cancer had grip weakness at diagnosis in the right (median [min–max], 9.5 (1.0–20.0) vs. 16.0 [7.0–34.6] kg, p = 0.039) and left (median [min–max], 9.2 [0.5–22.5] vs. 15.3 [6.8–33.6] kg, p = 0.042) hands prior to starting chemotherapy, compared to controls. Newly diagnosed patients also experienced progressive strength loss during induction therapy in both right (median [min–max], 11.4 [6.3–28.6] vs. 9.7 [1.0–20.0] kg, p < 0.001) and left (12.1 [4.3–33.1] vs. 9.7 [0.5–22.5] kg, p = 0.009) hands compared to their baseline measures, and took longer to complete the TUG test following the completion of induction therapy than they did prior to starting chemotherapy (median [min–max], 8.4 [6.9–10.2] vs. 11.0 [8.3–22.8] s, p = 0.008). These deficits improved following cessation of treatment. Off-therapy patients, compared to newly diagnosed patients, had significantly stronger grip strength in both right (median [min–max], 18.9 [8.0–37.6] vs. 9.5 [1.0–20.0] kg, p < 0.001) and left (19.4 [7.2–37.6] vs. 9.2 [0.5–22.5] kg, p < 0.001) hands, as well as faster TUG times (median [min–max], 6.8 [6.0–11.5] vs. 11.0 [8.3–22.8] s, p < 0.001). Unfortunately, off-therapy patients were still weaker and had impaired mobility compared to controls (children without cancer). These data suggest that impaired motor performance is related to diagnosis and acute treatment and has the potential to improve following therapy cessation, but does not completely recover.
These studies are supported by data from Schoenmaker et al.67 who documented severe muscle weakness and mobility problems (such as transfers, walking, and navigating stairs) in children treated for standard risk ALL or T-cell non-Hodgkin lymphoma. In 18 Dutch children, nine children (mean age: 8.7 [range, 1–16] years, 55.5% male) were treated according to a German Berlin, Frankfurt, Muenster (BFM)-based protocol (ALL-8), with the other nine children (mean age: 7.5 [range, 2–15] years, 44.4% male) treated on protocol ALL-9, an antimetabolite treatment high in dexamethasone and vincristine. MMT of the upper and lower extremities, scored by the criteria of the Medical Research Council, using a 6-point scale (grade range, 0–5),101 was conducted prospectively at time of diagnosis (T1 = week 0), twice during treatment (T2 = week 7 and T3 = week 28), at the end of treatment (T4 = week 105), and 6 months after completion of treatment (T5 = week 131). HHD was also used to evaluate isometric force of upper and lower extremity muscles on the nondominant side at diagnosis and 6 months after treatment. Investigators also measured functional skills, including mobility, with the adapted Dutch version of the Pediatric Evaluation of Disability Inventory (PEDI).102 In pooled analysis, muscle weakness and mobility issues were most severe in the first 2 months of treatment. Specifically, MMT revealed weakness in all patients (grade ≤ 4, “active movement,” against gravity with some resistance) in at least one muscle group, both proximally and distally, and was most apparent at 7 weeks following chemotherapy induction. At this same assessment, patients also presented with functional mobility problems scoring below the normal range (30–70 points) on the PEDI mobility scale. At 6 months after completion of treatment, MMT strength improved to a grade 5 (“normal power”) in 15 of 18 participants, although HHD revealed that muscle strength of the knee and foot extensors remained significantly decreased compared to reference values,103 again suggesting that impaired motor performance (weakness) persists after cessation of therapy.
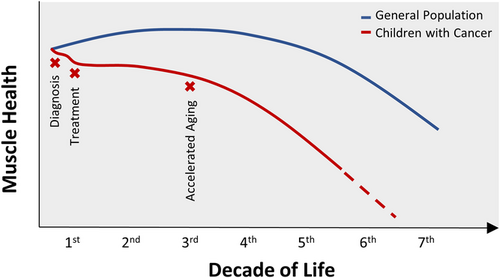
Overall, the sequence of events that contribute to low muscle mass and weakness in children with cancer is difficult to discern. This is in part due to limited investigations that assess muscle-centric outcomes, small samples sizes in existing investigations, differences in evaluation techniques, and different diagnostic populations studied. However, it is likely that muscle wasting and weakness begin early at diagnosis15-17, 30, 104, 105 and worsens with treatment (Figure 2).15, 16, 67, 96, 104 Although some children may partially recover their muscle mass,96, 104 muscle quality is likely compromised. Such, these children enter survivorship with poor muscle health, likely contributing to the early onset of chronic conditions.6, 7 Furthermore, survivors have muscle mass decreases independent of or preceding strength loss,71 which is unlike older adults without a history of childhood cancer who experience decreases in muscle strength preceding mass loss. Whether this pattern is present during childhood cancer treatment remains unclear. Thus, the interrelationship between mass and function in children with cancer deserves attention in future research to better understand the phenotype and develop strategies to limit its consequences.
3.2 Long-term outcomes
Impaired muscle quality or function following cancer treatment may contribute to poor function and reduced fitness in survivorship. Most investigations observing muscle outcomes in childhood cancer survivors have been conducted in childhood ALL survivors. Low lean mass is one of the most commonly reported outcomes in long-term ALL survivors.5, 24, 68-73, 106 Boland et al.69 observed lower relative (68.6% vs. 71.4%) lean mass and lower absolute (55.0 vs. 57.2 kg) lean muscle in childhood ALL survivors compared to age-, sex-, and race-matched controls. This study also noted that survivors (N = 365, median age: 28.5 [range, 23.6–31.7] years, 52% male, 87% White) are less likely to report participating in resistance training than controls (45.4% vs. 53.8%), suggesting that lifestyle choices may contribute to low muscle mass in this population.
The degree of muscle mass and strength loss in survivors can differ with previous treatment exposures. Tonorezos et al.68 evaluated the contribution of diet and physical activity to metabolic parameters among 117 long-term survivors of childhood cancer (median age: 23 [range, 18–37] years, 44% male), with and without a history of cranial radiation therapy (CRT). This study found that survivors with a history of CRT had lower lean body mass compared to survivors without CRT exposure (47.8 ± 12.4 vs. 52.7 ± 11.0 kg, p = 0.04) despite no difference in physical activity or total daily protein intake. We captured an association between CRT exposure and muscle strength in 75 young adults (mean age: 30.2 ± 7.1 years, 41.3% male, 98.7% White) previously treated for childhood ALL (mean diagnosis age: 5.6 ± 4.3 years).24 Using HHD to measure lower extremity strength, we found that males were 76.7 Newtons and females were 58.6 Newtons weaker on average in their knee extensor muscles than expected (expected males 569.87 N, females 464.67 N). Survivors exposed to CRT were weaker than survivors who were not exposed, although a significant association was only found among females. Further, knee extensor strength was positively correlated with percent skeletal muscle mass (r = 0.27, p = 0.02) assessed by DXA. Male survivors had 2.6% and females had 2.2% less muscle mass than expected (expected males 40.7%, females 32.3%).107-110 Treatment exposures such as CRT may contribute to tissue compositional changes within a muscle (i.e., contractile tissue vs. noncontractile tissue), influencing strength capabilities and muscle mass maintenance. However, it is unclear whether strength loss in survivors is due to an infiltration of fat or fibrotic tissue into skeletal muscle, and whether muscle mass is compromised as a result.
Muscle strength and flexibility are also known to be impaired following childhood cancer treatment and contribute to reduced mobility and walking efficiency. We clinically assessed neuromuscular impairments and physical performance limitations in 415 childhood ALL survivors (median age: 35 [range, 21–52] years, 48.9%, 97.3%) 10+ years from diagnosis (median time since diagnosis: 29.9 [range, 13.7–46.5] years).26 Knee extension strength and active dorsiflexion range of motion (ROM) were impaired in survivors (30.1% and 33.5%, respectively). In this study, survivors with impaired knee extension strength were 8.2 times (95% confidence interval [CI], 2.3–29.0) more likely to have limited mobility, and 2.3 times (95% CI, 1.4–4.1) more likely to have limited walking efficiency compared to survivors without impaired knee extensor strength. Survivors with ankle ROM deficits were more likely to have impaired walking efficiency (odds ratio [OR] = 3.9; 95% CI, 2.4–6.3) compared to those without ROM deficits. These findings are consistent with a study by Gerber et al.111 who assessed ROM, strength, and walking velocity in 32 survivors (mean age: 35.4 ± 10.6 years, 53.1%) of pediatric sarcoma. Walking velocity was positively correlated with ROM deficits (r = 0.50, p = 0.06) and strength loss (r = 0.74, p = 0.002) in female survivors, suggesting likelihood for reduced exercise tolerance and elevated risk for functional loss. Unlike survivors of childhood ALL, pediatric sarcoma survivors often receive surgical intervention as part of the treatment plan. Thus, they have added risk for muscular complications in survivorship that can influence their daily mobility and physical activity levels. A recent study by Marina et al.8 found that survivors who were surgically treated for lower extremity sarcomas during childhood cancer treatment had a 50% increased risk for activity limitations during survivorship compared to upper extremity sarcoma survivors.
Muscle endurance, the ability to offset fatigue, is an important aspect of muscle fitness. Poor muscle endurance manifests as a decreased capacity to do work and thus reduced efficiency of performance that normally follows a period of activity. As an indicator of muscle fitness, good endurance is evident in a quicker recovery from physical exertion and longer time to fatigue. Hovi et al.22 included an evaluation of muscle endurance in their strength assessments of 43 young female survivors of childhood ALL (mean age: 19 [range, 14–30] years) that were off-therapy (mean time: 8 [range, 1–19] years). Arm extension endurance was determined by maximum number of push-ups participants could complete within 1 min. Trunk flexion endurance was determined by the maximum number of sit-ups performed in 1 min. Performance numbers were compared to 69 healthy women (mean age: 19.4 [range, 14–30] years). The average number of completed repetitions for push-ups (7.2 [range, 2.0–12.4], p = 0.008) and sit-ups (5.8 [range, –0.2 to 11.8], p = 0.06) was significantly less in survivors compared to controls. This study also found muscle endurance to be associated with treatment exposures. Survivors exposed to radiation did, on average, 7.1 (95% CI, –1.5 to 15.8 reps, p = 0.10) fewer push-ups, and those exposed to asparaginase 7.6 (95% CI, 1.3–13.9, p = 0.019) fewer push-ups and 10.6 fewer sit-ups (95% CI, –4.3 to 16.9 reps, p = 0.002) than those not exposed. We also compared muscle endurance of the lower extremities in a large cohort of childhood ALL survivors (n = 365, median age: 28.5 [range, 23.6–31.7] years, 52% male, 87% White) with and without CRT exposure during cancer treatment, to age-, sex-, and race-matched controls.25 In this study, lower extremity muscle endurance was evaluated using an isokinetic dynamometer. In a seated position, participants performed repeated isokinetic knee extensions (Newton-meters [Nm]/kg at and 300°/s). Consistent with Hovi et al.,22 this study reported lower endurance in quadricep muscles in both CRT exposed (69.4 ± 24.2 vs. 93.1 ± 35.1 Nm/kg, p < 0.001) and non-CRT exposed (81.7 ± 30.5 vs. 93.1 ± 35.1 Nm/kg, 0 < 0.001) survivors compared to normative values. Muscle fatigue results from adenosine triphosphate depletion and is related to energy availability during repeated movement. Reduced muscle endurance suggests that mitochondrial function and/or substrate oxidation may be compromised in this population, translating to imitations in repeated or prolonged functional movements (i.e., walking and running), interfering with ability to fully participate in sport and/or recreational activities.
Muscle strength is the most commonly measured functional outcome in survivors of childhood cancer. Strength deficits often present concurrently with impaired exercise capacity as the skeletal muscle system is one of several organ systems that integrate functions during exercise. Van Brussel et al.28 evaluated physical function and fitness in 13 young survivors of childhood ALL (mean age: 15.5 ± 5.8 years, 46.2% male) who were within 5–6 years of chemotherapy cessation. On average, muscle strength determined by HHD was significantly lower in the knee extensor muscles of survivors compared to controls (252.1 ± 81.13 vs. 299.7 ± 98.9 kg, p = 0.001). Anerobic fitness was evaluated using a Wingate test,112 and aerobic fitness with a cardiopulmonary exercise test. Compared to controls, survivors, on average, generated lower peak power (538.6 ± 289.6 vs. 867.32 ± 508.2 W, p < 0.001) and mean power (376.1 ± 171.9 vs. 492.9 ± 276.9 W, p < 0.001) outputs. Survivors also had a lower exercise capacity on average than controls (VO2 peak, 36.64 ± 18.3 vs. 49.58 ± 21.22 ml/kg/min). Recently, we evaluated exercise intolerance, mortality, and organ system impairment in 1041 ten+ years survivors of childhood cancer (mean age: 35.6 ± 8.8 years, 49.3% male, 85.15% non-Hispanic White) and 285 community controls (mean age: 34.5 ± 10.0 years, 48.8% male, 90.2% non-Hispanic White),113 and found that a one standard deviation decrease in quadricep strength increased the odds of having an ejection fraction <53% (OR = 1.50; 95% CI, 1.30–1.72), and of having an exercise tolerance <85% of predicted (OR = 1.49; 95% CI, 1.23–1.82). These data indicate that continued monitoring of muscle fitness in childhood cancer survivors is important as reduced strength and aerobic fitness are associated with mortality in healthy aging individuals, and among adults undergoing cancer therapy.114-117
3.3 Accelerated aging in childhood cancer survivors
In the general population, muscle mass declines at a rate of 5% per decade beginning in the fourth decade, accelerating in the fifth and sixth decades of life, and is prevalent in both healthy and frail populations.118-120 Muscle strength also decreases by 10%–15% per decade of life until 70 years when losses are further accelerated.121, 122 Loss of muscle mass accounts for 5% of the change in strength. Together, the loss of muscle mass and strength are used to describe sarcopenia. Sarcopenia is a progressive and generalized skeletal muscle disorder with known adverse outcomes that includes both impaired function (low muscle strength) and structural damage (low mass/quality) as its primary diagnostic components. The presence of sarcopenia has been linked to increased risk for fall and fractures,123-125 impaired mobility126 and activities of daily living,127, 128 and loss of independence.129 It is further associated with cardiac disease,130 respiratory disease,131 and cognitive impairment,132 contributing to lowered quality of life133 and even death.134-136 This current operational definition of sarcopenia is an evolution from very early works that referenced decreased muscle mass with age as the sole indicator of sarcopenia.137, 138 Because it is now known that mass loss can occur independent of age, resultant from conditions such as cancer and malnutrition, the scientific community no longer utilizes muscle mass as the sole-defining parameter of sarcopenia. Although loss of muscle mass is still important, muscle strength is the best predictor of health outcomes and is the central diagnostic criteria of sarcopenia.139-141 Muscle quality and physical performance are indicators of sarcopenia severity.
Recently, childhood cancer survivors have been identified at risk for early onset of aging phenotypes that are heavily dependent on muscle mass and strength.28, 71, 24 Frailty, a phenotype that uses sarcopenia in its diagnostic criteria, is prevalent in 13.1% of young adult childhood cancer survivors (mean age: 33.6 ± 8.1 years, 50.3% male, 43.8% leukemia diagnosis).71 However, in this study, declines in muscle mass were independent of muscle strength, with some survivors demonstrating similar strength relative to sex- and age-matched controls, perhaps indicating that frailty differs among young survivors when compared to aging adults, who typically lose strength before they lose mass.142 In addition, it is not yet clear the exact nature and timing of muscle decline among children with cancer. Current evidence suggests that it begins early, emerges during cancer therapy, and can extend into survivorship immediately or reappear early in young adulthood.17, 24, 143, 144 Nevertheless, as in older populations, frailty in young adult survivors of childhood cancer is associated with new onset chronic disease and increased hazard of death over 3 years. It is a significant problem.
In adults, muscle protein is maintained by synchronized periods of net catabolism in post absorptive states and net anabolism in postprandial states,145 with balance in protein turnover closely correlated with fat-free mass. However, protein turnover declines with advancing age, even when adjusted for fat-free mass,146 suggesting that with age, synchrony between protein synthesis and proteolysis is altered or resistant. Results from a recent exercise and nutrition intervention suggest there is an underlying resistance to nutrition-stimulated anabolism in the muscle of childhood cancer survivors, similar to that seen in older adults. Krull et al.147 conducted a randomized placebo-controlled trial in 70 adult survivors of childhood cancer with low muscle mass. Participants were randomized to resistance training with protein supplement (21 g whey protein per day) (median age: 33.0 [range, 20.6–44.2] years, 55.2% male) or resistance training with placebo (sucrose) (median age: 33.7 [range, 21.1–44.9] years, 50.0% male) group. The training component consisted of whole-body resistance training, three times week for 24 weeks. At the end of the intervention, lean body mass improved in both groups (supplement: 1.05 ± 2.34 kg, p = 0.04; placebo: 0.13 ± 2.19 kg, p = 0.74; p = 0.11 for comparison of change between groups) compared to DXA baseline lean mass. This study demonstrated that resistance training supplemented by protein supplementation is not better than resistance training alone, and that response to resistance training is only moderate in adult survivors of childhood cancer with low lean muscle mass.
In addition to age-related decreased anabolic response and increased catabolic state, senescent skeletal muscle has impaired regenerative capacity.148 Among childhood cancer survivors, it is possible that treatment may damage or alter proteins, and contribute to slower tissue repair/recovery throughout life.149 Other potential mechanisms of age-associated declines in muscle quality and function include, but are not limited to, fiber atrophy and distribution,150-152 fat and fibrotic infiltration,153, 154 anabolic resistance and altered protein synthesis,155, 156 impaired metabolism and mitochondrial function,157, 158 neuromuscular remodeling,159, 160 oxidative damage,161, 162 regenerative impairment,163 and dysregulated cell maintenance.164-166 Aging-associated physiological impairments such as chronic inflammation, atherosclerosis, insulin resistance, and neurological deficiencies may also contribute to systemic decline.167-170 Specifically, chronic inflammation, the result of cellular damage incurred from aggressive multimodal treatments received early in life, and characterized by prolonged exposure to pro-inflammatory cytokines in systemic circulation, coincides with shortened leukocyte telomere length in childhood cancer survivors and increases risk for metabolic syndrome.171 We hypothesize that these conditions, experienced by survivors of childhood cancer much sooner than expected when compared to their peers,172 also contribute to early loss of muscle health.
4 CANCER- AND TREATMENT-RELATED BIOMECHANISMS
4.1 Drug therapy
Chemotherapy delivery in children with cancer is multimodal and includes agents that acutely impact neuromuscular control, protein synthesis, and mitochondrial function and that accelerate protein degradation. Although research activity is underway to incorporate host, treatment, and genetic predictors of treatment-related toxicities, chemotherapy administration is still required to achieve cure. Acute toxicities do not always resolve, leaving survivors at risk for poor muscle health later in life. Because children with cancer are exposed to multiple agents, toxicities that impact muscle health are likely at least additive.
Neurotoxic agents such as methotrexate and vincristine173 are exposures among children treated for cancer that contribute to long-term muscle impairments.14 Vincristine, a vinca alkaloid, induces mitotic arrest and cell death in lymphoid malignant cells by interfering with microtubule formation and mitotic spindle dynamics.174-176 Vincristine-induced peripheral neuropathy is a common side effect in children with cancer, typically manifesting as distal muscle weakness, absent reflexes, and impaired flexibility.26, 27 It is both dose dependent and associated with a homozygous T-allele in the CEP72 gene.177-181 Methotrexate is an antimetabolite that inhibits DNA synthesis and arrests cellular proliferation by competitively inhibiting dihydrofolate reductase activity.182-184 When it is administered directly to the central nervous system, or given in high enough doses to cross the blood brain barrier, it may interfere with folate homeostasis in normal tissue.185, 186 Cumulative doses of intrathecal methotrexate in the range of 215–225 mg/m2 are associated with increased risk of impaired strength and reduced ankle ROM in children treated for ALL.25, 26 Inadequate nervous system signals acutely reduce motor recruitment and limit muscle force production, which, over time, likely blunts muscle sensitivity to contractile anabolic stimuli that facilitates myofibril synthesis and muscle growth
L-Asparaginase is also implicated as a potential risk factor for reduced muscle health in survivors of childhood ALL, acute myeloid leukemia, and non-Hodgkin lymphoma. It is an enzyme that hydrolyzes asparagine, the alpha-amino acid that promotes cell proliferation in both healthy and cancerous cells. Leukemia cells have little to no expression of the enzyme responsible for synthesizing asparagine. Thus, L-asparaginase elicits its cytotoxic effect by decreasing asparagine levels in serum and cerebrospinal fluid, blocking biosynthesis of DNA and RNA, ultimately inhibiting cell profleration.187 Childhood cancer survivors with a cumulative dose of ≥120,000 IU/m experience muscle weakness22 and impaired flexibility during long-term survivorship.25 The exact mechanism underlying impaired muscle function with asparaginase exposure during childhood cancer treatment is currently unknown. Given that L-asparaginase has an inhibitory effect on protein synthesis in cancer cells,188, 189 muscle protein synthesis rates in muscle cells may also be compromised. Asparaginase also metabolizes glutamine, a key intramuscular amino acid involved in the regulation of muscle protein synthesis and breakdown.190 Prolonged asparaginase treatment may result in decreased muscle cell anabolic signaling191 and mRNA transcription and translation, resulting in reduced protein accumulation and cellular mass. Indirect actions of asparaginase may include increased muscle damage during combined treatment with glucocorticoids192, 193 or altered muscle mass homeostasis and quality (i.e., intramural adiposity) through impaired β-cell function and increased fat infiltration.194-196
Mitochondrial dysfunction is implicated in the pathophysiological progression of many diseases including sarcopenia. Childhood cancer survivors are exposed to therapies capable of inducing mitochondrial damage.197 Prolonged high-dose exposures to anthracycline antibiotics impair mitochondrial respiration and increase reactive oxygen species release in muscle,198 compromising metabolic function of muscle cells, contributing to loss of muscle cells199-203 and to impaired glucose metabolism.204, 205 Doxorubicin, a specific anthracycline, is associated with long-term loss, disruption, and disassembly of myofibrils, and with mitochondrial swelling. Because mitochondrial dysfunction is implicated as a driver of aging,206-208 treatment-induced mitochondrial dysfunction and myofibril disassembly may be responsible for premature physiological aging in childhood cancer survivors.71, 209-211
Corticosteroids are the most common cause of drug-induced myopathy, characterized by proximal weakness and associated with duration of therapy, cumulative drug dose, and drug type.212 Corticosteroids induce muscle atrophy by increasing protein degradation rates via ubiquitin–proteasome and autophagy–lysosome systems,213, 214 and by directly inhibiting the activation of the mechanistic target of rapamycin, the main cellular pathway regulating muscle protein synthesis.215 Muscle mass and strength loss during cancer treatment involves preferential atrophy of type II force generating glycolytic muscle fibers.216, 217 Among children or adults with cancer and among survivors, higher cumulative doses of corticosteroids and prolonged exposure increase risk for muscle wasting and weakness.218, 219 DeAnglelis et al.220 assessed the effect of corticosteroid dose on muscle health in adults (age range: 24–71 years) with non-Hodgkin lymphoma, and demonstrated that weakness was more prevalent in proximal extremity muscles among those treated with a high-dose schedule of dexamethasone (50 mg/day, 3 days/week), and that weakness progressed with prolonged treatment. Schoenmaker et al.67 also reported persistent proximal muscle weakness in pediatric patients of ALL and T-NHL treated with high doses of dexamethasone. In a randomized study, the United Kingdom Medical Research Council ALL97 evaluated survival benefit of dexamethasone compared with prednisolone for childhood ALL,221 reporting lower limb weakness in the quadriceps and/or glutei muscles among all children, and a fivefold higher incidence of upper arm weakness in those treated with dexamethasone compared to prednisolone (2.8% vs. 0.5%).
In adults, drug-induced myopathies primarily cause weakness in proximal muscles of the legs and arms.222 Whether this is consistent for children with cancer is unknown. Yang and Choi74 followed 30 children (mean age: 10.9 ± 3.8) newly diagnosed with cancer (hematologic malignancies n = 19, solid tumors n = 11) during their first year of cancer treatment. DXA scans showed only a significant difference in lower leg lean mass in patients compared to healthy controls. However, whether certain muscle fiber types (i.e., “slow” vs. “fast” twitch) or whole muscle groups consistently experience preferential wasting as a result of diagnosis or treatment is unknown. Given that low muscle mass is related to treatment intolerance, infection risk, and overall worse outcomes,30 there is a need for investigation into wasting specificity for children diagnosed with cancer.
4.2 Radiotherapy
Radiation exposure also increases risk for muscle impairment in childhood cancer survivors. Soft tissue fibrosis occurs in 80% of pediatric sarcoma patients treated with radiation therapy,223 and can also manifest as asymmetrical muscle growth and reduced ROM. Hypoplasia is associated with direct radiation exposure >20 Gy at a young age.225 Survivors treated with total body irradiation have lower muscle strength on average than survivors with no irradiation exposure in the upper arm (elbow flexion strength: 30 N; 95% CI, 0.3–59 N, p = 0.048), hand (grip strength: 181.7 N; 95% CI, 114.3–249.1 N, p < 0.001), and knee extensors (quadricep strength: 114 Nm; 95% CI, 50–179 N, p < 0.001).22 Childhood ALL survivors treated with CRT have weaker hand grip strength (38.4 ± 13.9 vs. 38.2 ± 12.3 kg, p = 0.02), quadricep strength (158.3 ± 54.7 vs. 182.5 ± 57.1 Nm/kg, p < 0.001), and muscular endurance (69.4 ± 24.2 vs. 81.7 ± 30.5 Nm/kg, p = 0.002) than survivors without CRT exposure.25 In addition, female survivors of childhood ALL treated with CRT have lower fat-free (%) and relative lean mass (g/m2) (58.7 ± 6.2% and 18.4 ± 3.0 g/m2) when compared to survivors without CRT exposure (66.0 ± 7.5% and 17.2 ± 2.9 g/m2) and age- and sex-matched controls (65.1 ± 8.5% and 17.5 ± 3.7 g/m2).
Although hormonal dysregulation is largely responsible for reduced lean mass in childhood cancer survivors exposed to cranial radiation,20, 24, 70, 224, 226-229 the exact mechanism responsible for muscle impairment has not been studied extensively in children whose muscle tissue is directly exposed to radiation during therapy. However, damage to muscle satellite cells (SCs) is likely. SCs are responsible for early muscle development, muscle growth in response to external stimuli (e.g., resistance training),230 and muscle repair following myocyte injury. Information from animal studies indicates that acute radiation exposure at doses of radiation ≥5 Gy reduces muscle SC number by 70% in young adult rats,231 muscle exposed to radiation fails to regain pre-radiation myonuclei count and DNA content in response to overloading,232 and fibro/adipogenic progenitor cells necessary for initial phases of SC-mediated muscle repair fail to adequately clear from the regenerative niche.233, 234 As such, further research is needed to elucidate the acute effects of radiation on muscle SCs in children with cancer, and the long-term implications on muscle health during survivorship.
Currently it is unknown whether drug- or radiotherapy-induced muscle wasting in children with cancer is reversible. This is primarily due to the limited knowledge of whether the biological mechanisms discussed above are similar among children with cancer. However, epidemiological evidence supports that children with low lean mass at or before diagnosis have continually low lean mass during treatment.88 Children with cancer do not seem to return to pretreatment muscle mass amounts, nor recover to the amount of muscle mass of their healthy peers.74, 96 Given limited access to skeletal muscle tissue in children, there is a need for future research to incorporate molecular imaging techniques and animal modeling methods to begin to answer these important mechanistic questions.
5 INTERVENTIONS TO IMPROVE MUSCLE HEALTH
Interventions during primary cancer treatment have had mixed success in improving muscle function. One of the largest investigations was by Morales et al.235 who evaluated the efficacy of exercise training in 24 children (mean age: 10 ± 4 years) with solid tumors (NCT01645436). Children completed aerobic and strength exercise sessions three times a week over the 19 ± 8 weeks intervention period, spanning chemotherapy treatment. Maximum muscle strength was evaluated by a 5 rep-maximum test on pediatric-specific weight training machines.236 Compared to baseline, muscle strength across all muscle groups (chest, back, legs) improved (p < 0.01). However, no significant improvements in functional performance on the TUG and timed up and down stairs (TUDS) tests was observed. In a smaller sample of patients, Keats and Culos-Reed237 conducted a physical activity intervention in 10 adolescents (mean age: 16.2 ± 1.6 years) with mixed diagnoses (lymphoma n = 4, leukemia n = 4, CNS tumor n = 1, germ cell tumor n = 1). Over the 16-week intervention, participants performed 45 min of aerobic and 15 min of strength and flexibility training. Significant improvements in upper body strength, indicated by more push-ups completed on the 90° push-up test,238 were observed as early as 8 weeks into the intervention.
Physical therapy is also capable of improving muscle function in this population. Marchese et al.239 evaluated the effects of 4 months of physical therapy compared to no physical therapy in 28 children (age range: 4–18 years) with ALL. The intervention consisted of three sessions of supervised physical therapy and an individualized home exercise program that combined functional, strength, flexibility, and aerobic exercise 5 days a week for 12 weeks during maintenance therapy. After 4 months, both ankle dorsiflexion and knee extension strength measured by HHD improved significantly, compared to controls. Preoperative physical therapy sessions have also proven to be beneficial in maintaining or restoring physical function in children with lower extremity malignancies. In a prospective clinical trial (NCT01674101 ),19 14 children and adolescents (mean age: 13.5 ± 3.5 years) with lower extremity malignancies completed 60-min physical therapy sessions focused on endurance, strength, and flexibility three times a week for 10 weeks prior to local control. Physical function was evaluated after 10–12 weeks of preoperative physical therapy, and at 20–22 weeks after local control using the Functional Mobility Assessment,240 a break test via HHD to assess lower extremity strength and a goniometer to quantify joint ROM. The patients who received the preoperative physical therapy had better mobility reflected in significantly higher scores on the Functional Mobility Assessment than controls (35.6 ± 10.3 vs. 25.7 ± 13.7 points, p = 0.0267). However, the intervention did not result in strength improvements in the lower limbs. This suggests that either the training intensity was insufficient in activating the physiologic pathways contributing to strength gains or preoperative chemotherapy limits contraction capability and/or results in a resistance to neuromuscular adaptations.
Collectively, these studies re-enforce that the effect of childhood cancer on muscle function is complex, and that physical function can improve despite continued weakness. These studies also highlight that changes in muscle mass are not evaluated alongside changes in muscle function. In fact, we are unaware of any clinical trial directed at mitigating muscle wasting in children with cancer. Given that muscle mass is a key criterion of sarcopenia and is protective against treatment-associated toxicity, there is a need to evaluate whether interventions administered during treatment can rescue muscle mass losses in this population. However, without an understanding of the pathobiological mechanisms driving these losses, it is difficult to develop such interventions.
6 SUMMARY
In the year 2020, it is estimated that 15,590 children and adolescents under the age of 19 will be diagnosed with cancer.241 In survivorship, these children will be burdened by long-term morbidity and mortality associated with late-term treatment effects; nearly two thirds of childhood cancer survivors have at least one chronic health condition 30 years after diagnosis.6 Muscle is implicated in many of these conditions and loss of mass and strength continues to be reported.5, 20, 22-24, 67 Children with cancer often have poor muscle health and function that can progressively worsen with treatment, persisting into survivorship, increasing risk for reduced physiologic reserve, insulin resistance, and exercise intolerance. Epidemiological evidence to support poor muscle health as an adverse outcome of childhood cancer is strong; weakness, muscle wasting, peripheral neuropathy, poor muscle endurance, and impaired flexibility and functional mobility are commonly reported across diagnoses. Further, key chemotherapy (methotrexate, vincristine, L-asparaginase, doxorubicin, corticosteroids) and radiation (total body, cranial) exposures have both been associated with long-term impairments in muscle mass and function. Biomechanistic investigations have identified key biological processes altered by cancer therapy; mitochondrial dysfunction, satellite cell damage, hormone dysregulation, impaired cellular anabolism, altered cell cycle, and neurophysiologic dysfunction can result in compromised muscle. We hypothesize that these biological mechanisms are altered in children with cancer and contribute to poor muscle following treatment, accelerating the onset of premature physiological aging and chronic health conditions during survivorship. Future research is needed to evaluate these mechanisms in the children with cancer in order to design and implement targeted lifestyle and therapeutic interventions directed to improving muscle health and quality of life across the childhood cancer continuum.
ACKNOWLEDGMENTS
Financial support for this work was provided by a Cancer Center Support Grant (P30CA021765, Charles Roberts) from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC). The authors would like to acknowledge Tracie Gatewood for her assistance with article preparation.
AUTHOR CONTRIBUTIONS
CGG and KKN contributed to the conceptualization, investigation, writing, and editing. REP and KKN contributed to the review and editing. KKN provided supervision.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




