Contributions of Yap and Taz dysfunction to breast cancer initiation, progression, and aging-related susceptibility
Abstract
Yap and Taz are co-transcription factors that have been implicated in the development of many cancers. Here, we review the literature that analyzes the function of Yap/Taz in normal breast and breast cancer contexts. Our review of the literature suggests that Yap and Taz are involved in breast cancer and Taz, in particular, is involved in the triple-negative subtype. Nevertheless, the precise contexts in which Yap/Taz contribute to specific breast cancer phenotypes remains unclear. Indeed, Yap/Taz dysregulation acts differentially and in multiple epithelial cell types during early breast cancer progression. We propose Yap/Taz activation promotes breast cancer phenotypes in breast cancer precursor cells. Further, Yap dysregulation as a result of aging in breast tissue may result in microenvironments that increase the fitness of breast cancer precursor cells relative to the normal epithelia.
1 INTRODUCTION
Yap and Taz co-transcription factors initiate new transcriptional programs in a variety of cellular contexts (Figure 1). Subsequently, we will refer to both transcription factors as “Yap/Taz” when the described conditions are known to apply to both. The Yap/Taz ortholog Yorkie was originally discovered in the fruit fly, downstream of the Hippo signaling pathway, where it causes cell proliferation as a part of normal tissue size control during development.1 Since then, Yap and Taz have both been shown to be activated in a variety of contexts including: perturbed cell polarity, extracellular stiffness, low cell density, wound healing, regeneration, embryonic development, and stem cell maintenance (Figure 1).2-15 In addition, Yap/Taz can be activated by other cell signaling events including signaling through Wnts, glucocorticoid receptors, G-protein coupled receptors, and PI3K signaling.16-22 Yap/Taz induced transcription causes many different cellular phenotypes, such as cell growth, apoptosis, stem cell renewal, and cellular differentiation (Figure 1).12-15 With their ability to activate proliferation it is no surprise that Yap/Taz are upregulated in many diseases, including cancers (Figure 1). Some of the cancers in which Yap/Taz are involved include: breast, lung, colorectal, liver, gastric, and pancreatic.12, 23, 24 In most of these contexts Yap/Taz are implicated in tumor growth and metastasis. However, in some cases Yap/Taz also are involved in drug resistance and cancer stem cell phenotypes.12
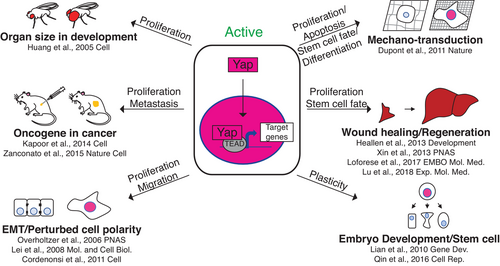
The goal of this review is to take a focused perspective on the function of Yap and Taz in normal breast and breast cancer tissues. This review will be broken into three parts: (i) the role of Yap/Taz in a normal breast context, (ii) the role of Yap/Taz in a breast cancer context, and (iii) the differential roles that Yap/Taz may play in separate breast epithelial cell types to promote breast cancer during the earliest stages of progression.
2 YAP AND TAZ IN NORMAL BREAST TISSUE
At first glance, the mammary gland is comprised of two main cell layers. The outer, or basal-located, layer contains contractile myoepithelial cells whereas the inner cell layer is composed of secretory luminal epithelial cells (Figure 2A).25 Most human studies suggest Yap/Taz functions in the basal cell layer of breast epithelium because Yap/Taz protein is primarily localized in the nuclei of myoepithelial cells (Figure 2B; Table 1).4, 26-34 Furthermore, genes that typically change in response to Yap/Taz upregulation (ie, Yap/Taz transcriptional signatures) are detected in the RNA expression profiles of myoepithelial cells and mammary stem cells.32, 35 In general, it is thought that Yap/Taz normally functions to maintain the stem cell population during high rates of proliferation in non-homeostatic conditions such as wound healing, regeneration, and cancer.15 The mammary gland goes through many cycles of heavy proliferation throughout a woman's lifetime, including early development, puberty, menstruation, and pregnancy.36, 37 Therefore, it is reasonable to speculate that Yap/Taz may function similarly in mammary epithelium to maintain a stem cell fate during periods of high proliferation.
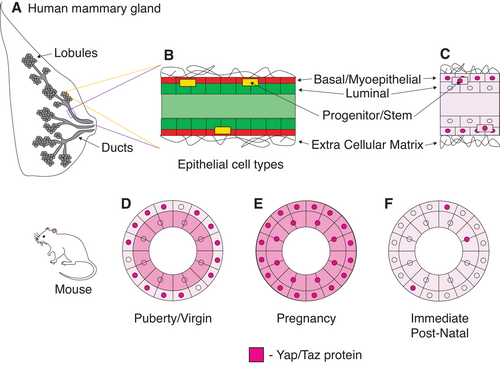
Studies in mice suggest Yap/Taz functions dynamically in mammary epithelium depending on contexts that require high rates of proliferation (Figure 2D-F and Table 1). For example, Taz protein accumulates in the nuclei of myoepithelial cells during puberty and is necessary for the maintenance of the myoepithelial cell lineage throughout this period of massive tissue re-structuring and growth (Figure 2D).32 The localization of Yap protein changes drastically surrounding pregnancy, a period in which mammary tissue is heavily remodeled. Prior to pregnancy, Yap accumulates in the nuclei of myoepithelial cells (Figure 2D). During pregnancy, Yap additionally localizes in the nuclei of luminal cells and functions to promote the survival of progenitor cells (Figure 2E). And after pregnancy, Yap expression is significantly decreased in both cell types (Figure 2F).38 Although, Yap/Taz accumulation has not been looked at during pregnancy in humans it is feasible that they perform similar functions. These studies in mammary epithelial tissues of humans and mice support the hypothesis that Yap/Taz generally functions to maintain a progenitor cell fate during periods of high proliferation. However, it is not clear what are the precise molecular changes that Yap/Taz induce during these periods and if they vary during different proliferative events (i.e. development, puberty, menstruation, and pregnancy). Moreover, a number of different myoepithelial, luminal, and progenitor cell subtypes have been identified in the breast epithelium,39-42 and it is important to consider that Yap/Taz may confer distinct functions in these different epithelial cell subtypes.
3 YAP AND TAZ IN BREAST CANCER
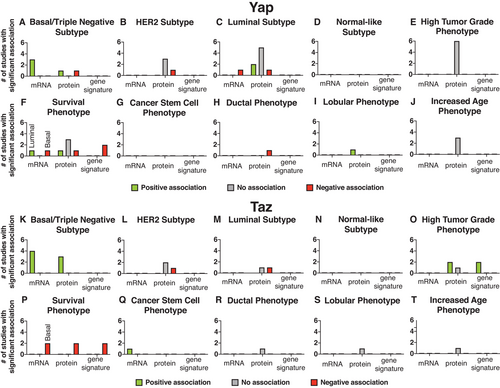
Many studies have examined the expression of Yap/Taz in different in vivo breast cancer contexts to determine whether and how Yap/Taz contributes to breast cancer. Here, we summarize the results of such studies that have looked at Yap/Taz mRNA expression, protein expression, or transcriptional signatures in specific breast cancer subtypes or phenotypes (Figure 3; Tables 2-4). Taz expression positively correlates with the triple-negative subtype and cancer stem cell phenotype (Figure 3K,O,Q).35, 43-45 Consistent with involvement in the triple-negative subtype, it is estimated that Taz gene amplifications occur in 6.5-44% of basal tumors (Table 9).2, 4, 32, 43 Yap/Taz expression was significantly associated, mainly a negative correlation, with breast cancer survival in a number of studies and is, therefore, highly likely to be involved in breast cancer progression (Figure 3F,P).4, 24, 32, 33, 35, 43-48 However, our review of literature indicates that more studies need to be done to define the precise environments in which Yap/Taz contributes to breast cancer. One common theme we see in our analysis is the seemingly contradictory evidence about Yap/Taz involvement in any particular breast cancer subtype or phenotype. For example, different studies conclude that Yap is upregulated, downregulated, or does not change in association with the luminal subtype (Figure 3C).26, 27, 33, 46-48 This suggests either that (i) some of the methods used in these studies do not accurately reflect Yap/Taz activation and/or (ii) that Yap/Taz play differential roles within particular breast cancer subtypes or phenotypes and more work is necessary to decipher the precise contexts in which Yap/Taz actually acts.
| Subtype/Phenotype | Analysis | Correlation | Reference |
|---|---|---|---|
| Basal/Triple Negative Subtype |
RNA Protein |
+ + + + − |
|
| Her2 Subtype | Protein |
− None None None |
|
| Luminal Subtype |
RNA Protein |
− + + (PR) − None None None None None (ER) |
|
| High Tumor Grade Phenotype | Protein |
None None None None None None |
|
| Survival Phenotype |
RNA Protein Gene Signature |
+ (Luminal) − (Basal) + (dis. free) − (overall) None (5 yr) None (dis. free) None (overall) − − |
|
| Ductal Phenotype | Protein | − | 29 |
| Lobular Phenotype | Protein | + | 29 |
| Increased Age Phenotype | Protein |
None None None |
| Subtype/Phenotype | Analysis | Correlation | Reference |
|---|---|---|---|
| Basal/Triple Negative Subtype |
RNA Protein |
+ + + + + + + |
|
| Her2 Subtype | Protein |
− None None |
|
| Luminal Subtype | Protein |
− None |
|
| High Tumor Grade Phenotype |
Protein Gene Signature |
+ + None + + |
|
| Survival Phenotype |
RNA Protein Gene Signature |
− (basal) − (basal) − − − − |
|
| Cancer Stem Cell Phenotype | RNA | + | 45 |
| Ductal Phenotype | Protein | None | 43 |
| Lobular Phenotype | Protein | None | 43 |
| Increased Age Phenotype | Protein | None | 43 |
| Reference | Scoring Method |
|---|---|
| 26 | +/− Yap |
| 34 | OE Taz |
| 4 | + Taz (> 10% of tumor cells had nuclear staining similar or greater than nearby normal cells in same section) |
| 48 | Yap 0–3 in BC tissue |
| 27 | Yap +/−, intensity, location |
| 28 | Yap -, similar, + compared to normal tissue |
| 29 | Nuclear Yap Low/High (20%) |
| 30 | Yap + |
| 47 | N Yap + (10% cells moderately expressing) |
| 46 | +/− (moderate staining in > 10% of tumor cells) Yap |
| 45 | Taz 0–3 (1-3 compared to normal ducts in same section) |
| 43 | Taz 0–3 (based on % of + N staining) |
| 35 | +/− (scoring system) |
| 35 | +/− (> 10 % of tumor had moderate staining) |
| 35 | N Yap/Taz +/− |
| 44 | N Taz +/− |
| 33 | Yap +/− (score) |
In support of the first scenario, we note that these studies used drastically different methods (eg, antibodies, concentrations, and scoring methods) to come to their conclusions (Table 4). Yap/Taz expression itself also does not necessarily translate to increased transcriptional function. We suggest future studies use methods that more accurately measure Yap/Taz activation such as: nuclear localization and/or transcriptional signatures. Indeed, emerging studies that analyze Yap/Taz transcriptional signatures have uncovered some contexts in which Yap/Taz contribute to breast cancer: high tumor grade, cells that have mutated p53 activity, and cells that have high glucocorticoid receptor activity.4, 18, 24, 49
In support of the second scenario, we note there are some contexts in which Yap/Taz function has not been thoroughly assessed. We suggest Yap/Taz may act at an earlier time point during cancer progression; these studies primarily looked at Yap/Taz expression in cancers that have already formed. To this end, Yap/Taz function should be compared between young normal epithelial tissues and tissues that have an increased risk to become cancerous including: BRCA mutant, aged, hyperplasia, and DCIS tissues. In addition, Yap/Taz may have diverse roles that contribute to breast cancer in different epithelial cell types. For example, Yap/Taz may be upregulated or downregulated differentially in myoepithelial, progenitor, and luminal cells and cause different phenotypes that collectively lead to an increase in breast cancer development. We propose that future studies in diverse breast epithelial cell types and at different points in breast cancer progression, using methods that accurately measure Yap/Taz function, will improve our knowledge of when Yap/Taz acts during breast cancer development.
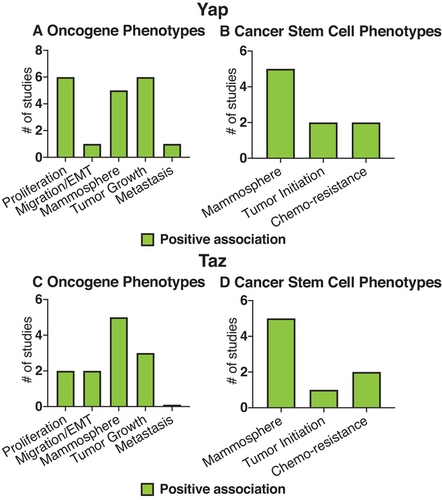
Review of the literature in vitro further suggests Yap/Taz are involved in breast cancer (Figure 4). Cell culture studies showed that Yap/Taz contribute to breast cancer-relevant cell culture phenotypes including: proliferation, migration, anchorage-independent growth, tumor growth, and metastasis (Figure 4A,C) (Table 5, 6).2-4, 18, 24, 28, 32, 34, 35, 38, 44, 49-51 These studies also found that Yap/Taz induced breast cancer stem cell phenotypes, such as mammosphere initiation, tumor initiation, and chemo-resistance (Figure 4B,D; Table 7).2, 4, 18, 24, 32, 35, 44 In addition, Yap/Taz were generally found to be upregulated in basal/triple-negative cell lines compared to cell lines that represent Her2+ or luminal breast cancer subtypes (Table 8).32, 35, 43, 52 However, in our analysis of this literature, we notice that a majority of the studies, especially the ones that analyze Taz function and a cancer stem cell phenotype, look at a limited subset of breast epithelial cell lines (mainly MCF10A, which were derived from human fibrocystic breast tissue; Tables 5-7). Also, some limitations exist by only looking at Yap/Taz function in cell lines. Cell lines, by definition, are already immortal, and therefore exclude the analysis of Yap/Taz function during the earliest stages of breast cancer progression, prior to immortalization. Evidence suggests Yap/Taz function differentially depending on immortalization. For example, mechanically triggered Yap/Taz activity in human mammary epithelial cells (HMEC) from postmenopausal women requires exposure to super-physiological matrix stiffness (>3 GPa) and results in lineage-specific proliferation patterns, whereas the isogenic immortalized HMEC cell lines trigger Yap/Taz at physiological stiffness (1000-2500 Pa) and cause proliferation in all lineages.31 In addition, cell lines likely do not represent the majority of epithelial cell types in which Yap/Taz could function. We reiterate that future cell culture studies in diverse epithelial cell types and during earlier time points during breast cancer progression would greatly expand our knowledge of Yap/Taz involvement in breast cancer.
| Reference | Cell line | Perturbation | Cancer phenotype |
|---|---|---|---|
| 2 | MCF10A | Up | Migration, EMT, Proliferation, AIG |
| 50 | Human cell lines (4) | Up | Tumor growth and metastasis in mice |
| 28 |
MCF7 410.4 (mouse) |
Down Up |
Decreased cell and tumor growth Cell and tumor growth |
| 51 |
MCF10A SW527 |
Down Down |
Decreased cell proliferation and survival Decreased cell proliferation, AIG, survival, and xenograft growth |
| 38 |
PyMT (mouse) Human cell lines (8) |
Down Down |
Decreased tumor growth Decreased cell growth |
| 35 | MCF10A | Up | Mammosphere formation, tumor initiation |
| 24 | MDA-MB-231 | Down | Decreased cell growth, mammosphere formation, tumor initiation |
| 49 | SK-Br-3 | Down | Decreased cell growth |
| 18 | MCF10A-M2 |
Down Up |
Decreased mammosphere formation Mammosphere formation |
| Reference | Cell line | Perturbation | Cancer phenotype |
|---|---|---|---|
| 34 |
MCF10A MCF7 |
Up Down |
Migration Decreased Migration, AIG, and tumor growth |
| 3 | MCF10A | Up | Cell growth, EMT, Migration |
| 4 |
MCF10A-T1K- M2 (Ras) MCF10A-CA1a- M4 (Malig. deriv.) |
Down Up Down |
Decreased mammosphere formation Mammosphere formation, tumorigenesis Decreased mammosphere formation, decreased tumorigenesis |
| 32 | MCF10A | Down | Decreased mammosphere formation |
| 44 | MCF10A | Up | Mammosphere formation, tumorigenesis |
| 24 | MDA-MB-231 | Down | Decreased cell growth and mammosphere formation |
| Reference | Yap/Taz | Tissue/Cell line | Cancer stem cell phenotype |
|---|---|---|---|
|
Taz Taz Yap Taz Yap/Taz Yap |
MCF10A- M2 + M4 MCF10A MCF10A MCF10A MCF10A + M2 MCF10A- M2 |
Mammosphere formation | |
|
Taz Yap Yap |
MCF10A- M4 4T1 MCF10A |
Tumor initiation | |
|
Yap Taz Taz Yap |
MCF10A MCF10A- M2 Human BCSC MCF10A- M2 |
Chemo-resistance |
| Reference | Cell line subtypes | Finding |
|---|---|---|
| 32 | Luminal (5), Basal (4) | Taz upregulated in basal cell lines |
| 43 | Luminal (2), Her2 (2), Basal (2) | Taz upregulated in basal cell lines |
| 35 | Her2 (3), Luminal (4), Basal (6) | Yap upregulated in basal cell lines |
| 52 | Basal (5), non-basal (5) | Yap upregulated in basal cell lines |
| Reference | Tissue/Cell line | Type of amplification |
|---|---|---|
| 2 | Mouse tumor (Brca1 −/− and p53 +/−) | Yap gene is amplified |
| 4 | Tumors | Taz gene amplified in 27/313 tumors |
| 32 | TCGA | Taz copy no. amplification in 44% of basal subtype |
| 43 |
TCGA BC (FISH analyses) |
Taz high copy gain in 7.4% of basal subtype Taz FISH amplification in 6.5% of TN subtype |
| 79 | BC tissue |
Loss of heterozygosity in region that contains Yap gene. 39% (22/57) |
| 80 | BC tissue |
Loss of heterozygosity in region that contains Yap gene. 43% (19/44) |
| 81 | BC tissue |
Loss of heterozygosity in region that contains Yap gene. 37–43% (44-51/120) |
| 26 | BC tissue |
Loss of heterozygosity in region that contains Yap gene. 33% (9/27) |
Indeed, many studies seemingly contradict each other in regard to Yap/Taz involvement in breast cancer. Yap/Taz expression has been concluded to correlate negatively, positively, and not at all with the same breast cancer subtype, depending on the study (Figure 3). In addition, Yap/Taz is often viewed as a tumor-driving villain, whereas Yap/Taz apparently also can act as tumor suppressors in some contexts.26, 53, 54 It is likely that Yap/Taz have differential tumor suppressor or oncogenic activities depending on the cell in which they are activated. For example, Yap/Taz were shown to act as tumor suppressors in normal liver cells and as oncogenes in liver cancer cells.55 This further exacerbates the need to study the function of Yap/Taz in different breast epithelial cell types during different stages of cancer progression to properly elucidate the role of Yap/Taz in breast cancer.
4 YAP AND TAZ IN DIVERSE BREAST EPITHELIAL CELL TYPES DURING THE EARLIEST STAGES OF PROGRESSION
Our analysis of the Yap/Taz literature in different breast epithelial cell types prior to immortalization has led us to the following three hypotheses:
4.1 Hypothesis 1: Yap/Taz dysfunction acts in the earliest stages of breast cancer progression
The earliest stages of breast cancer progression have been relatively understudied in the past, particularly because a majority of breast epithelial cells studied in culture have already undergone many steps of malignant progression. However, most of the oncogenes present in fulminant cancers also are detected earlier in progression, in pre-malignant ductal carcinoma in situ (DCIS) cancer precursor cells. An approach to studying Yap/Taz in earlier stages of breast cancer progression could take advantage of accurate cell culture models of breast cancer progression that start with normal HMEC from women that have undergone breast reduction surgery (Figure 5). The cell culture model that was developed by Martha Stampfer accurately reflects the earliest phases of breast cancer progression that occur in vivo, and supports the growth of different subtypes of normal breast epithelial cells, including: myoepithelial, luminal, and progenitor cells.56-62 This model has been used to good effect to study early events in breast cancer progression and the roles of putative oncogenes that promote breast cancer development.
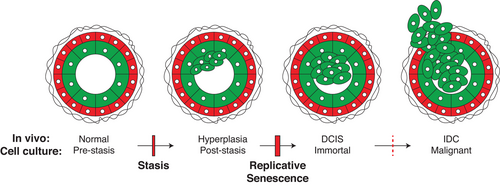
Aging is arguably the most significant risk factor for the development of breast cancer.63, 64 We previously proposed that specific molecular features associated with an aging phenotype act in breast cancer precursor cells and contribute to early breast cancer progression.65 In normal HMEC the subcellular localization and function of Yap changes as a function of age (Figure 6).31, 42 Therefore, Yap is a good candidate protein whose regulation and function changes during the aging process and may contribute early on to the development of breast cancer, perhaps before there is anything recognizable as a pathological lesion. To further test this hypothesis, it will be important to perturb Yap signaling in HMEC from different aged specimens and assay for changes in oncogenic and early progression phenotypes such as proliferation, malignant properties, or barrier bypass.
4.2 Hypothesis 2: Yap/Taz activation may promote breast cancer phenotypes in luminal progenitor cells or luminal cells and lead them on a path towards cancer progression
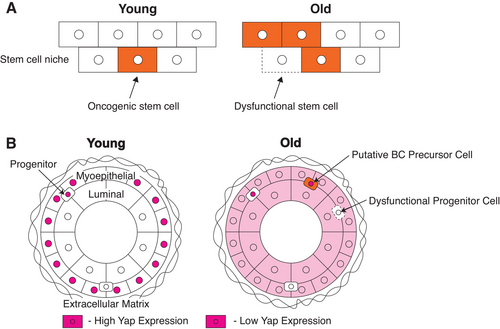
Most of the studies we reviewed consistently found that Yap/Taz activation is associated with oncogenic phenotypes (Figures 1 and 4 and Tables 5-7).12 Therefore, it is reasonable to speculate that Yap/Taz is activated in the subset of mammary epithelial cells that are thought to give rise to cancer. Luminal-biased progenitor cells are thought to be the cell of origin for most breast cancers.66 Alternatively, it also has been proposed that Yap/Taz activation in non-progenitor cell types could cause them to take on sufficient stem cell properties that could lead them on a path to cancer development.67 By protein analysis, we have found a subset of dysfunctional luminal progenitor cells that increase with age, which likely model a breast cancer cell of origin in culture (Figure 6).42 Significantly, Yap was expressed and active in this cell type42 (Figure 6B, orange and pink). Future studies perturbing Yap in this in vitro model of breast cancer-cells-of-origin followed by an analysis of oncogenic and early progression phenotypes will be important to elucidate Yap's role in early breast cancer progression. Yap also was shown to activate transcription of hTERT,68, 69 thus it is plausible that Yap may contribute to immortalization in breast cancer precursor cells in addition to causing malignancy and/or stem cell phenotypes early in progression. That speculation is particularly tempting in the context of age-related breast cancers. Yap was detected in the cytoplasm and nuclei of luminal epithelia cells in healthy breast tissue from women in their early 50s.31 Perhaps a low level of dysregulated Yap-dependent hTERT expression causes cells residing in the luminal neighborhood (including luminal-biased progenitors) to become more susceptible to malignant transformation by enabling more ready bypass of the replicative senescence barrier (Figure 5).
4.3 Hypothesis 3: Yap/Taz dysregulation in non-cancer precursor cells as a function of age may promote an environment permissive for cancer progression
The adaptive oncogenesis hypothesis suggests that cancer arises when an oncogenic stem cell gains a selective advantage in dividing over non-oncogenic stem cells that occupy the same microenvironment. Therefore, cancer progresses when the non-oncogenic cells that contribute to the stem cell niche have a general decrease in fitness, such as insults that occur as a natural part of the aging process70 (Figure 6A).
Many cell types likely contribute to the breast epithelial microenvironment in which breast cancer progenitor cells reside. A subset of dysfunctional luminal progenitor cells is thought to be the oncogenic stem cells that progress to cancer and these are part of a microenvironment that is composed of myoepithelial cells, luminal epithelial cells, and other progenitor cells.66, 71 Therefore, the relative fitness of the different lineages and progenitor cells likely contribute to a breast cancer precursor cell's ability to become cancerous (Figure 6B). The myoepithelial layer is generally thought of as a barrier to cancer.72, 73 Indeed, the fitness or age of myoepithelial cells has a direct effect on gene expression and DNA methylation in the luminal layer as shown by the use of primary cell-derived bilayer tissue cultures. Young pre-menopausal luminal cells grown on older post-menopausal myoepithelial cells have decreased expression of genes that uniquely contribute to luminal-lineage specificity.71 This suggests dysfunctional or aged myoepithelial cells change the fitness of nearby cells, which may create a microenvironment where breast cancer precursor cells will have greater fitness relative to normal cells. Consistent with this concept, dysfunctional mammary epithelial progenitor cells accumulate with age.74 The decreased fitness of dysfunctional mammary epithelial progenitor cells also may create a microenvironment permissive for the continued growth of oncogenic breast cancer precursor cells. We postulate that the relative fitness of luminal, myoepithelial, and progenitor cells as a function of age likely creates a microenvironment that affects the ability of breast cancer precursor cells to progress towards cancer. Testing such a hypothesis could include perturbing Yap signaling in HMEC from the different aged specimens, performing bilayer co-culture experiments, and assaying for changes in the fitness of different progenitor cell types.
Yap function itself becomes dysregulated in a few different non-cancer precursor cell types with age (Figure 6B). (a) In vivo, Yap starts to accumulate in the cytoplasm and nuclei of luminal cells with age, which suggests Yap may become active in this cell type as women age31 (Figure 6B, Luminal). Given that the breast microenvironment changes with age,65 Yap is activated in response to specific microenvironments (ie, stiffness),5 and Yap induces the transcription of molecules that further induce a stiff microenvironment.75 Thus, a feed-forward Yap activation loop could be engaged in a subset of aged cells that ultimately contributes to an altered, unfit microenvironment. It is possible that this altered microenvironment can further cause gene expression changes that contribute to aging phenotypes and decreased fitness in surrounding cells. (b) In vivo, Yap also changes subcellular localization in myoepithelial cells with age, such that Yap additionally accumulates in the cytoplasm31 (Figure 6B, Myoepithelial). This may further indicate an altered expression/function of Yap with age in myoepithelial cells that could contribute to a decrease in tissue fitness. For example, if myoepithelial cells are not able to properly activate Yap in response to microenvironment cues they may not be able to perform their normal barrier function. (c) Yap function is dysregulated in progenitor cells as a function of the aging process as well (Figure 6B, dashed line). Yap activation in response to stiffness in young progenitor cells causes these cells to differentiate in favor of a more myoepithelial cell fate compared to a luminal one.31 Because the myoepithlia performs an anticancer barrier function, this may contribute to the age-bias development of breast cancer. Proper Yap activation in young progenitor cells causes changes that inhibit breast cancer progression. In contrast, Yap is not properly activated in response to stiffness in older progenitor cells.31 Therefore, older breast tissues are not able to respond and may not be able to protect themselves in response to extracellular stiffness cues. As a result, this could create an environment permissive for oncogenic progenitor cells to thrive and become cancerous, especially if these cancer precursors are capable of normal Yap activation and proliferate in response to an older stiffer microenvironment. A mathematical model that incorporates age-dependent changes in the ability of the tissue to repress malignant growth predicted that malignant cells with a high mutation rate and few initial cancer driver genes will cause tumors at a younger age, whereas malignant cells with multiple drivers and low mutation rates will cause tumors later in life.76 Consistent with that concept, basal breast cancers have fewer drivers with high mutation rates and infamously inflict younger women, whereas luminal subtype breast cancers are genetically heterogeneous with low mutation rates and are dominantly age-related.77, 78 Yap dysregulation with age in noncancer precursor cells may be an essential event promoting a microenvironment permissive for breast cancer progression. To further explore this hypothesis, one could perturb Yap in different HMEC cell types from different aged specimens and test for changes in relative fitness.
5 SUMMARY/FUTURE OUTLOOKS
Here, we take a focused look at Yap/Taz function in breast cancer. Our analysis of the literature agrees with the interpretation that Yap/Taz are involved in breast cancer and that Taz in particular is associated with the basal/triple-negative subtype in fulminant cancers. Furthermore, the literature in total supports the concept that Yap/Taz allow access to cell functions needed for stereotypical malignant behavior and cancer stem cell states, such as EMT genes. However, we also notice that much more information is needed to understand and further elucidate the precise timing, location, and function of Yap/Taz in diverse mammary epithelial cell contexts and its relationship to the development of breast cancer.
To this end, the function of Yap has been studied in a cell culture model of early breast cancer progression, starting with normal finite cells. These studies have led us to hypothesize that Yap dysregulation may act at the earliest stages of breast cancer progression. In addition, Yap may play different roles in distinct mammary epithelial cell types that altogether leads to the early progression of breast cancer. If Yap/Taz contributes to breast cancer phenotypes at the earliest stages of progression, this may lead to the development of novel therapeutics that are better aimed to prevent breast cancer. One can develop therapeutics that can stop either Yap/Taz themselves or some of their downstream transcriptional targets that contribute to Yap/Taz-induced early cancer phenotypes.
ACKNOWLEDGMENTS
We thank our patient advocates Susan Samson and Sandy Preto for their valuable contributions to our research team. We are grateful for support from our sponsors: NIH F32CA247311 to TF; and CDMRP BCRP Era of Hope Award (BC141351) and associated expansion (BC181737), NIH (R01EB024989, U54HG008100), Conrad N. Hilton Foundation, and City of Hope Center for Cancer and Aging to ML.
AUTHOR CONTRIBUTIONS
TF and ML contributed to conceptualization, investigation, writing and editing, and funding acquisition. ML provided supervision.
CONFLICTS OF INTEREST
TF and ML have no conflicts of interest to declare




