High concentrations of fluorescent nanoprobes delayed Oryzias melastigma embryo hatching by modulating respiratory and metabolic pathways
Tianle Tang and Jinyan Chen contributed equally to this work.
Abstract
A new graphene-based fluorescent nanoprobe for tumor cell nucleus (GTTNs) was synthesized in our previous study that penetrates the tumor cell membrane and particularly targets cancer cell nucleus and displays tremendous potential for clinical applications. As part of the biocompatibility assessment, we have previously evaluated GTTN for in vivo acute and subacute toxicity, as well as reproductive toxicity. However, the potential developmental toxicity of GTTN remains unexplored, which is essential for its clinical application. In the present study, we exposed Oryzias melastigma embryos to different concentrations of GTTN (0, 100, 500, 1000, 2000, and 3000 mg/L). The results revealed that only the highest concentration (3000 mg/L) caused a significant reduction in the embryo hatching rate, with no observable effects on heartrate, behavior, or juvenile pathology. To further investigate the underlying cause of the delayed hatching, we conducted a series of experiments, including embryonic eukaryotic (single-cell) RNA sequencing, scanning electron microscopy imaging of the embryo surface, and embryo surface washing. The findings indicated that GTTN exhibited favorable biocompatibility, even at the highest concentration (3000 mg/L). The reason for the delayed embryo hatching is not the toxicity of GTTN itself, but the continuous adsorption of GTTN on the surface of the embryo from the beginning, which affects its respiration and metabolism, and further affecting its growth and development. These provide valuable information and guidance for the application of GTTNs in biological and medical fields.
1 INTRODUCTION
Nanomaterials and their functionalized derivatives have developed rapidly in the past few decades.1 Due to their small size and excellent physicochemical properties, nanomaterials such as graphene quantum dots, carbon dots (CDs), carbon nanotubes, silica nanoparticles, and gold nanoparticles have great potential applications in biomedicine, including areas such as cancer diagnosis and therapy, drug delivery, and bioimaging.2 However, the efficient tumor targeting and biocompatibility of nanomaterials with potential clinical applications in tumor diagnosis and therapy have been a great clinical challenge.3 Nanomedicine has been reported to show a low targeting rate of only 5% at the tumor site based on the traditional targeting method of enhanced permeability and retention (EPR) effect.4 At the same time, the toxicity and biocompatibility of some nanomaterials have attracted much attention. Chen et al. found that Cu(OH)2 nanopesticides mediated neurodevelopmental delay and neuroactivity decline in zebrafish through multiple neurotransmitter receptor inhibition.5 One study reported that low concentrations (150 µg/mL) of CDs did not induce any evident embryonic toxicity or cause any apparent teratogenic effects during hatching or development. However, significant effects were observed in zebrafish embryos at CDs concentrations of >200 µg/mL, including pericardial and yolk sac edema, delayed growth, spinal cord flexure, and death, which were further associated with the reduction in zebrafish larval locomotor activity and decreased dopamine levels, reduced frequencies of tyrosine hydroxylase-positive dopaminergic neurons, and multiple organ damage.6
In a previous study, we developed a novel graphene-based tumor cell nuclear targeting fluorescent nanoprobe (GTTN) with a graphene-like single-crystalline structure using a modified base-mediated hydrothermal molecular fusion method.7 With the characteristic of specific tumor cell targeting, the GTTN directly crosses the cell membrane and specifically targets the tumor cell nucleus. Notably, with a cell membrane permeability targeting mechanism, the GTTN improves the tumor-targeting rate from less than 5% of the EPR effect to greater than 50%.4, 7 These excellent characteristics indicate that the GTTN has great potential for tumor diagnosis and treatment. In addition, we confirmed that GTTNs exposure had little cytotoxicity in vitro, acute and subacute toxicity in vivo and good biocompatibility in mice through CCK-8 in vitro cell assay and in vivo experiments on behavior, organ coefficient, body weight, histological examination, and biochemical indicators.8 Furthermore, reproductive toxicity evaluation studies of GTTN on reproduction and offspring health demonstrated that GTTNs-exposed male mice retained good fertility, healthy structure of testes and epididymides, and production of healthy sperm.9 Undoubtedly, the findings provide valuable information for the toxicological studies and biomedical applications of GTTN. However, the developmental toxicity of GTTN has not been studied, which is essential for its clinical application.
In this study, Oryzias melastigma embryos were exposed to different concentrations of GTTN (0, 100, 500, 1000, 2000, 3000 mg/L); it was found that only the highest concentration of GTTN caused a significant difference in embryo hatching rate, but no significant difference in heartbeat, behavioral, and juvenile pathological sections. In order to further explore the cause of incubation delay, we carried out a series of experiments, such as embryonic eukaryotic (single-cell) RNA sequencing, scanning electron microscope (SEM) imaging of the embryo surface, and washing the embryo surface, to analyze and explain the toxicity mechanism of the highest concentration of GTTN (Figure 1A). These provide valuable information and guidance for the application of GTTNs in biological and medical fields.
2 RESULTS
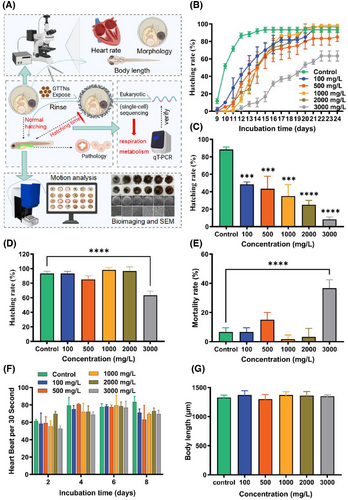
2.1 GTTN exposed to O. melastigma embryos
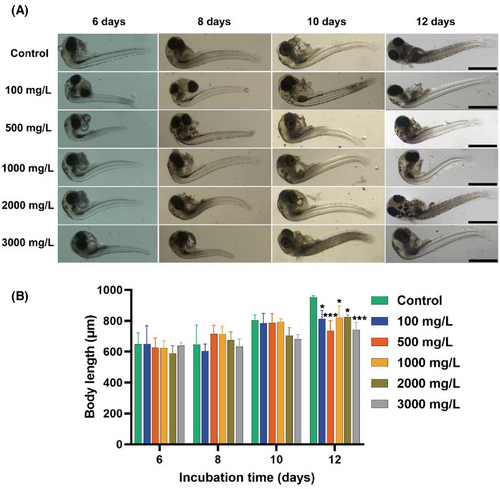
The GTTN prepared in the laboratory exhibits ideal water solubility and fluorescence properties, and it is crucial to evaluate its toxicity using relevant vertebrate models. We treated the O. melastigma embryos with different concentrations of GTTN solution (0, 100, 500, 1000, 2000, 3000 mg/L), and the end point of the experiment was embryo hatching or death. The results showed that the incubation delay of embryos in the experimental group was obvious after exposure, especially in the group with the highest concentration of 3000 mg/L (Figure 1B). On day 13, the hatchability of each group was 88.33%, 48.33%, 43.33%, 35%, 25%, and 8.33%, respectively (Figure 1C). Interestingly, at the end of the experiment (embryo hatching or death), the hatching rates were 93.33%, 93.33%, 85%, 98.33%, 96.67%, and 63.33%, respectively. Only the 3000 mg/L group showed significant differences (p < .0001) (Figure 1D). The mortality rate was also the same, with only a significant difference between the highest concentration group of 3000 mg/L and the control group (p < .0001) (Figure 1E). After exposure to 2, 4, 6, and 8 days, the embryos of the O. melastigma showed no significant heart rate differences (Figure 1F). In addition, posthatching analysis revealed no significant differences in larval body length between any experimental groups and control group (Figure 1G).
2.2 Morphology
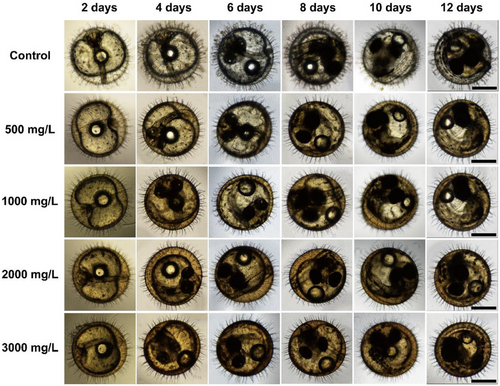
To understand the development and morphology of O. melastigma embryos at different time stages after exposure to different concentrations of GTTN. We exposed the embryos of O. melastigma to GTTN (0, 500, 1000, 2000, 3000 mg/L) and observed and recorded the embryo conditions on 2, 4, 6, 8, 10, and 12 days. According to the images, there were no obvious abnormalities or differences in the embryos (Figure 2).
2.3 Fluorescence observation and pathological sectioning
After exposure to different concentrations of GTTN (0, 500, 1000, 2000, 3000 mg/L) (Figure 3A,B), fluorescence observation was conducted on the hatched juvenile fish at 0, 12, and 24 h (Figure 3C–E). Initial observations at hatching onset showed predominant GTTN accumulation in intestinal and hepatic tissues. Subsequent analysis demonstrated rapid metabolic clearance, with only trace fluorescence detectable after 24 h.
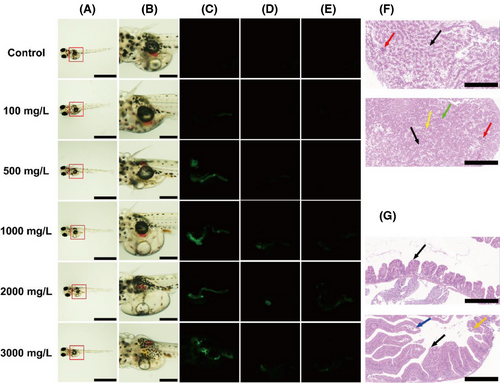
The histology of the aforementioned organs in O. melastigma treated with GTTNs was evaluated by fixing representative (fluorescent concentration area) organ samples from the treatment and control groups and staining them with hematoxylin and eosin (H&E). The intestinal tissue shows the mucosal layer folding into the intestinal lumen to form villi, which are finger shaped or club shaped, with folds formed at the folds. The mucosal layer is composed of mucosal epithelium and lamina propria. The mucosal epithelium is a single layer of columnar epithelium, and scattered goblet cells (black arrow) can be seen between the columnar epithelial cells. The lamina propria is composed of connective tissue, which is relatively thin, and the muscle fibers of the muscle layer are uniformly colored without obvious pathological changes (Figure 3F). Hepatic analysis revealed extensive hydropic degeneration, evidenced by cytoplasmic pallor and structural loosening (black arrows), though no significant necrosis or inflammatory infiltration was detected (Figure 3G).
2.4 Mechanical dechorionation experiment
Embryonic growth dynamics and developmental progression were quantitatively assessed through longitudinal body length measurements, which serves as a primary developmental metric. For this purpose, we want to obtain the changes in the development of the experimental group embryos by peeling off the embryonic chorion according to the physical shedding method (Figure 4A). On days 6, 8, 10, and 12, after shedding the chorion, comparative analysis revealed that dechorionated embryos maintained structural integrity with no significant teratogenic manifestations. However, the body length of the experimental group was lower than that of the control group on day 12, and development was affected (Figure 4B).
2.5 Motion analysis
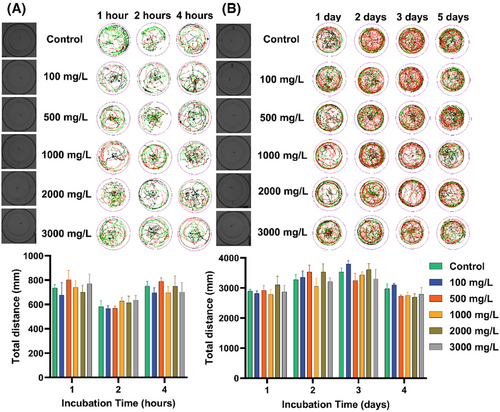
To understand the behavioral and neurological effects of GTTN exposure on juvenile O. melastigma. After incubation, there was no significant difference in the statistical analysis of the movement trajectory and total distance after exposure to 1, 2, and 4 h (Figure 5A). In addition, there was no significant difference in the experimental results including the movement trajectory and the total distance when we were exposed for 1, 2, 3, and 5 days (Figure 5B). These findings indicate that GTTN exposure does not significantly impair motor function or induce detectable neurobehavioral alterations of O. melastigma.
2.6 Observation of embryonic surface
We tracked and observed the changes on the surface of embryos after exposure to GTTN. From the beginning, flocculent substances continuously accumulated on the surface of embryos. After exposure for 14 days, the surface of embryos showed a significant increase in adhesive substances with the increase of GTTN concentration without rinsing. After rinsing it, it will be observed that there is no excess substance residue on the surface (Figure 6A). SEM observation shows that there are also a large amount of substances on the surface of the embryo after exposure without rinsing (Figure 6B). In the flushing experiment, the hatching rate curve of the control group did not show significant changes, but after flushing with a concentration of 3000 mg/L, the hatching rate of the control group increased significantly and showed almost no difference compared to the hatching rate of the control group (Figure 6C,D).
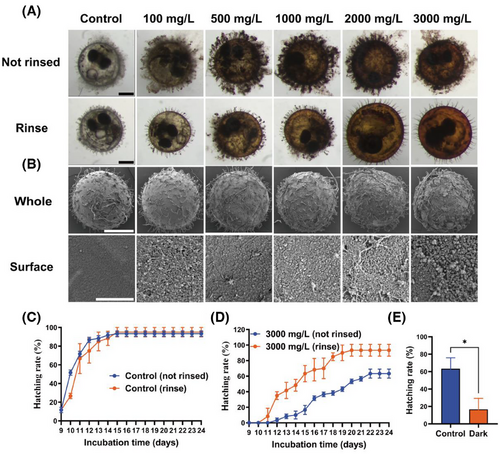
GTTN itself has color, and as the concentration gradually increases, the color also gradually deepens (Figure S1). Considering that dark colors can affect the light conditions of embryos (Tables S1 and S2), we conducted a dark experiment to observe the effect of light opacity on embryo hatching rate. The experimental results show that opacity can also significantly reduce the hatching rate of embryos (Figure 6E).
2.7 Eukaryotic (single-cell) sequencing and RT-qPCR validation
As the false discovery rate (FDR) value decreases, the difference in gene expression becomes significant. FDR and log2FC were used to screen differential genes, and the screening conditions were FDR < 0.05 and |log2FC| > 2. As shown in Figure 7A, 155, 231, 181, 216, and 806 differentially expressed genes (DEGs) were up-regulated and 290, 494, 606, 406, and 1206 DEGs were down-regulated in the 0, 500, 1000, 2000, and 3000 mg/L of GTTN groups, respectively.
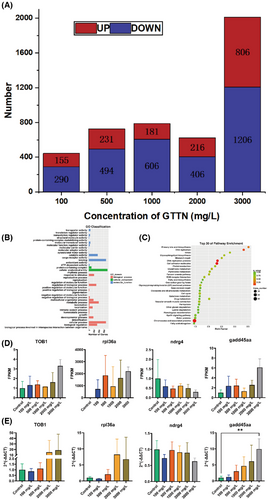
Transcripts from three Gene Ontology functional categories were annotated: molecular functions, biological processes, and cellular components. In the 3000 mg/L concentration group molecular function category, binding (1036 transcripts), catalytic activity (539 transcripts), and transporter activity (104 transcripts) are the most highly annotated small functional categories. Among the cell component categories, the most highly annotated is cellular anatomical entity (1127 transcripts), protein-containing complex (166 transcripts). While in the biological process category, cellular process (1032 transcripts), metabolic process (596 transcripts), and biological regulation (503 transcripts) are the most highly annotated small functional categories (Figure 7B).
Kyoto Encyclopedia of Genes and Genomes enrichment analysis was performed for 1039 DEGs at 3000 mg/L GTTN and control group (Figure 7C). The results showed that chromosome and associated proteins (115 DEGs, 11.07%), cell adhesion molecules (28 DEGs, 2.69%), DNA replication proteins (21 DEGs, 2.02%), and DNA replication (8 DEGs, 0.77%) were significantly enriched, and all of them were related to growth and development. This suggests that GTTN exposure may affect the growth and development of embryos.
To validate the transcriptome findings, we performed RT-qPCR analysis on four growth-related genes rpl36a, gadd45aa, TOB1, and ndrg4. The expression patterns across experimental groups demonstrated concordance between transcriptomic data and qPCR results, confirming the reliability of our sequencing data (Figure 7D,E).
3 DISCUSSION
The different concentrations (0, 100, 500, 1000, 2000, and 3000 mg/L) of GTTN was exposed to O. melastigma embryos. After exposure, heart rate, body length after incubation, and morphology did not change, and the experimental group showed incubation delay at 13 days. Interestingly, even at the end point, the 100, 500, 1000, and 2000 mg/L of GTTN groups were not significantly different from the control group, and only the highest concentration was 3000 mg/L. Many low-toxicity nanomaterials had similar results, with no significant toxicity in the low-concentration group and no serious toxicity in the high-concentration group, which only affected incubation.10 In this study, GTTNs exposed O. melastigma embryos, so we followed up to explore the mechanism of delayed hatching and reduced hatchability of O. melastigma embryos.
In subsequent experiments, we observed through fluorescence microscopy that GTTN mainly aggregates in the intestines and livers of newly hatched juvenile fish after exposure. We performed pathological sections and H&E staining on the intestines and livers of juvenile fish, and found no significant pathological changes in the intestines and livers. We continuously observed the fluorescence at 0, 12, and 24 h and found that after 24 h, the fluorescence almost disappeared, indicating that GTTN has been almost completely metabolized.
At the beginning of GTTN exposed embryos, the embryo surface adsorbed to GTTN over time. It has been shown that the nanoparticles would block the pores and prevent gas exchange and nitrogen waste, thus affecting the development of embryos.11 Meanwhile, when we measured the length after physical dechorion, we also showed that the control group was longer than the experimental group at 12 days, which affected the development. Therefore, we carried out the washing experiment, that is, washing the material adsorbed to the embryo surface once a day, the record found that the hatching rate of the experimental group was rescued, and at the end of the experiment, the hatching rate was basically not significantly different from that of the control group. In order to understand the effects of GTTN exposure on the mobility and neurotoxicity of juvenile medaka, we recorded the movement trajectory and total mileage statistics of juvenile fish after exposure to 1, 2, and 4 h and 1, 2, 3, and 5 days, and did not find significant differences. Therefore, GTTN did not affect the mobility and nervous system of developing juvenile fish. GTTN itself has color, and as the concentration increases, the color becomes darker, and the adhesion surface will further affect the embryo's light absorption. Numerous studies have shown that the lack of light in embryos affects the control of normal hatching rhythms and development through factors such as the pineal gland.12
After the exposure began, we observed continuous adhesion of substances on the surface of the embryo. We observed the embryo surface using a microscope and SEM, and found that there were indeed a large number of substances on the embryo surface. In order to verify whether the adhesion substances on the surface of embryos affect embryo hatching, we conducted a washing experiment. After washing off the surface substances of embryos every day and continuing to expose them, we found a significant improvement in embryo hatching rate. The experimental endpoint was even the same as the hatching rate of the control group. To further illustrate the effect of GTTN on embryos, we performed eukaryotic (single-cell) sequencing on a single embryo exposed for 14 days as a sample. Compared with the control group, the differential genes in the 3000 mg/L GTTN group were mainly related to growth, development and respiratory metabolism, including up-regulated expression of TOB1, rpl36a, and gadd45aa and down-regulated expression of ndrg4.
After exposure to GTTN, a large number of ribosomal protein (rpl36a) genes undergo changes. Ribosomal proteins form most cellular proteins and are essential for cell growth. The mutation of ribosomal protein is also associated with tissue-specific phenotype, indicating that it may play a role in organ development during early embryonic development.13 Gadd45aa protein acts as a tumor suppressor and stress sensor, playing a key role in genotoxic and non-genotoxic stress responses. Upon DNA damage, Gadd45aa expression is rapidly induced, leading to cell cycle arrest and/or apoptosis. P53 regulates these genes through transcription, which either induce cell cycle arrest (G1-S and G2-M), leading to DNA repair, or induce apoptosis by inducing pro-apoptotic genes.14 TOB1 is a member of the TOB/BTG antiproliferative protein family, and previous studies have shown that this family of proteins participates in the regulation of tissue growth and development by negatively regulating the cell cycle. The TOB family proteins are expressed in the blastocyst and gastrulation stages of zebrafish, indicating their role in early embryogenesis. In the late stage of embryonic development, the TOB gene is expressed in the notochord, hatching gland, blood island, and intestine of fish.15 The results of eukaryotic (single-cell) sequencing experiments showed that TOB1 was upregulated, delaying the proliferation and development of embryos. Ndrg4, also known as BDM1 and SMAP-8, are one of the four members of the N-Myc downstream regulatory gene (NDRG) family, which are involved in cell proliferation, differentiation, development, and stress. NDRG4 is expressed in the embryonic heart and central nervous system (CNS). Knocking down ndrg4 in zebrafish embryos significantly reduces the proliferation of muscle cells and leads to poor cardiac development.16
The concentration of this experiment is much higher than that of cell imaging and animal experiments, so in the range of use and environmental concentration, GTTN has little effect and is almost nontoxic. Due to the adhesion of surface substances affecting embryonic respiration and material exchange, and then the embryo hatchability rate decreases, and the hatchability rate is seriously inhibited.17 In the end, we considered the morphological characteristics of the surface of the bluefin tuna eggs, as observed under a microscope, which showed numerous fuzzy hairs on the surface of the eggs. In addition, the bluefin tuna eggs were attached to the female's abdomen through sticky filaments. These special surface structures give fish eggs a dual adhesion mechanism of morphology and biochemistry. In addition, GTTN has an extremely small size, with a lateral dimension of only 2.6–4.1 nm (average 3.35–0.15 nm) and a high specific surface area, resulting in a relatively high surface energy. This makes it easy for GTTN to accumulate in large quantities on the surface of bluefin tuna eggs, leading to an increase in hatching time.17, 18
4 CONCLUSION
In this study, we investigated the toxic effects of GTTN on O. melastigma embryonic development. Oryzias melastigma embryos were exposed to different concentrations of GTTN (0, 100, 500, 1000, 2000, and 3000 mg/L), and it was found that only the highest concentration of GTTN caused a significant difference in embryo hatching rate, but there were no significant differences in heartbeat, behavior, and juvenile pathological sections compared with the control group. Further exploring the reasons for the delayed incubation caused by high concentration of GTTN, we found that the delayed embryo hatching is not the toxicity of GTTN itself, but the continuous adsorption of GTTN on the surface of the embryo from the beginning, which affects its respiration and metabolism, and further affecting its growth and development. This provides valuable information and guidance for the application of GTTN in biological and medical fields.
5 MATERIALS AND METHODS
5.1 Synthesis of GTTN
GTTN were synthesized according to the method previously described by our research group.7a
5.2 O. melastigma farming
In this study, O. melastigma was used as the test organism. Oryzias melastigma has been regarded as a model species for toxicology due to its small individuals, short generation time, high rate of spawning, and wide range of salinity adaptation.19 In addition, their embryos are transparent for direct observation of organ development and toxic phenotypes, and their genome is already fully annotated for genomic and bioinformatic analyses.20 All O. melastigma strains used in this study were wild-type animals, and all growth, maintenance, reproduction, embryo collection, and culture protocols were performed in a standard manner. Oryzias melastigma was fed with young shrimp (brine worm larvae, INVE), once a day, feeding fish with compound feed, twice a day. The culture temperature was maintained at 26–28°C, the salinity of artificial seawater was 30%, the pH was 8.2–8.4, and the light and dark cycle for 14 h: 10 h. The above environmental parameters of O. melastigma embryos exposed to GTTN remained unchanged throughout the experiment. Oryzias melastigma embryos were harvested by siphon the following morning and screened (∼34 h) in an open field with an inverted fluorescence microscope (DP-80 Cellsens, Olympus).
5.3 Exposure and toxicity analysis of GTTN in O. melastigma embryos
The control group was artificial seawater without other substances, and the experimental groups were 100, 500, 1000, 2000, and 3000 mg/L of GTTN solution, respectively. We change the exposure solution once a day. Each group of 20 embryos was exposed to a 96-well plate for approximately 34 h for three biological replicates. Embryo phenotypes were observed by inverted fluorescence microscope on the 2nd, 5th, 8th and 11th day after exposure, and embryo hatching rate, survival rate, mortality rate, body length, and heart rate were detected. Heart rate trials were recorded in the camera at a magnification of 10 objective.
5.4 Body length measurement experiment
After exposing O. melastigma embryos with different concentrations of GTTN (0, 100, 500, 1000, 2000, and 3000 mg/L), two pairs of high-precision straight tipped tweezers (China EZ5889, microscope instrument) were used to physically remove the chorion from the (6th, 8th, 10th, and 12th) day after exposure. One pair of tweezers was used to fix the embryo, while the second pair of tweezers was used to puncture the chorion, open the chorion, expand the fissure, and remove the chorion of the embryo (Figure S2). Take a photo and use ImageJ to measure the distance from the center of the eye to the tip of the tail bud as the body length of the fish.
5.5 Imaging of O. melastigma embryos and larvae exposed to the GTTN
Morphology was observed 2, 4, 6, 8, 10, 12, and 14 days using an inverted fluorescence embryonic microscope after exposure to concentration gradient GTTN. Newly hatched larvae (15 days) were visualized using a macro zoom microscopes (MVX 10+DP74, Olympus).
5.6 Histopathological analysis
Oryzias melastigma were exposed to a range of GTTN concentrations (0, 100, 500, 1000, 2000, and 3000 mg/L) for up to 14 days, and brain, gill, liver, and intestinal tissues were collected and fixed with 4% paraformaldehyde for 48 h. Paraffin section dewaxed to water, pretreatment, hematoxylin staining, eosin staining, desealing, microscope microscopy, image acquisition, and analysis. Reagents were provided by Wuhan Seville Company.
5.7 Movement analysis
Oryzias melastigma larvae were exposed to GTTN (0, 100, 500, 1000, 2000, and 3000 mg/L) for 1, 2, 3, and 5 days, after which they were transferred to 24-well plates and placed in Zebra Box to automatically track the fish and generate movement routes using Zebra Lab. The device was used to measure the distance (in mm) of O. melastigma within 30 min (10 min–10 min dark–10 min).
5.8 Rinsing experiments
Early O. melastigma embryos is divided into two groups, in the process of concentration gradient GTTN exposed O. melastigma embryos, a group of daily after washing to continue into GTTN solution, a group of no washing, both groups will be changed every day and statistical incubation rate, heart rate, microscope morphology observation, using view variable fluorescence microscope and dissecting microscope (Zeiss) observation before and after washing embryo surface material attachment.
5.9 The dark experiment
The screened O. melastigma embryos were placed into a transparent 96-well plate (control group), and the experimental group put the embryos into a 96-well plate (BS-MP-96B, Biosharp), replaced the artificial seawater every day, observed the hatching of fish eggs every day and made statistics.
5.10 Eukaryotic (single-cell) sequencing
Expose approximately 34 h of O. melastigma embryos to a bacterial culture dish (BS-90-D, Biosharp) containing 50 mL (0, 100, 500, 1000, 2000, and 3000 mg/L) of GTTNs solution. The bacterial plates were washed on time every day, and the solution was replaced. Embryos were collected at day 13 after exposure, 2 mL sterile enzyme-free cell (BS-20-ST55, Biosharp), load dry ice transport to Shanghai Biotechnology Co., Ltd for eukaryotic (single-cell) RNA sequencing. There are a total of 18 trace Total RNA samples in this project, which were analyzed after using SMART Seq® Kit amplification, cDNA was obtained and subjected to Qubit and Agilent Bioanalyzer 2100 (Agilent Technologies) electrophoresis quality inspection before being used for future reference. Amplify single-cell samples or perform cDNA fragmentation, end repair, 3ʹ end A addition, linker ligation, enrichment, and other steps to complete the construction of sequencing sample libraries. The library built uses Qubit® 2.0 Fluorometer detects concentration, Agilent 2100 detects library size. Use Illumina NOVA6000 sequencer and select PE150 mode for sequencing. The data volume is about 6 G per sample, and the proportion of bases with a mass greater than 20 (Q20) in each direction is not less than 85%. The O. melastigma transcriptome was sequenced using the Illumina sequencing platform with 48–79.87 million filtered reads per sample with Q20 (percent phr quality score > 20) values greater than 95%. The results show that the samples and data are of high quality and enable subsequent experiments and analysis. Typically, a gene is considered differentially expressed if it is more than twice differentially expressed in two sets of samples.
5.11 RT-qPCR validation
RT-qPCR experiment was performed on the embryos of the O. melastigma after exposure to GTTNs (0, 100, 500, 1000, 2000, and 3000 mg/L) for 13 days, with the aim of confirming the accuracy of eukaryotic (single-cell) sequencing results. Total RNA from each set of individual O. melastigma embryos was reverse transcribed using the GoScript reverse transcription system (A5001, Promega) following the manufacturer's instructions. The RT-qPCR amplification was performed using Long TL 988-IV (Xian), and three biological and three technical replicates of all genes in each pool using GoTaq RT-qPCR Master Mix (A6002, Promega). The reaction profile is as follows: 95°C initial denaturation for 10 min, 95°C denaturation for 15s, 60°C annealing/extension for 60s, 40 cycles, and GADPH as the housekeeping gene. RT-qPCR was performed on TOB1, rpl36a, gadd45aa, and ndrg4 to observe the expression levels (Table 1). The relative mRNA expression levels of antioxidant response-related genes were analyzed with the method of 2−∆∆Ct.
| Test gene primer names | Primer sequences |
|---|---|
| OM-GADPH-F | CGGTTGTCAGCAATGCATCC |
| OM-GADPH-R | ACTGTGCTCATCAAGCCCTC |
| TOB1-F | CAGCAGGAACGGACACGAC |
| TOB1-R | TTCGGTCCAGGATCATCGAGGT |
| rpl36a-F | TCCCTCTATGCACAGGGTAAGA |
| rpl36a-R | TTGGATCTGCAGTTGGGCTC |
| gadd45aa-F | ATGGACTCGGTAGCCAAAGC |
| gadd45aa-R | ACGTTATCCGGGTCTACGTTT |
| ndrg4-F | CCGAGTCAAACTCCGAACAA |
| ndrg4-R | GTCTCAGAGTCCACGCCTCT |
5.12 Statistical analysis
All results are presented in the form of mean ± standard deviation (SD), and GraphPad Prism 9.5.0 was used for plotting and data analysis. First, Shapiro–Wilk test and Brown–Forsythe test were conducted, combined with Q–Q plot, and the results showed that the data conform to normal distribution and homogeneity of variance. Subsequently, hatching rate, mortality rate, posthatching body length, dark experiments, and RT-qPCR validation experiments were statistically analyzed using one-way ANOVA (and nonparametric); two-way ANOVA was used for statistical analysis of heart rate, body length measured by shedding of chorionic villi, and total behavioral distance. Dunnett's multiple comparison test will be conducted for all cases. *p < .05, **p < .01, ***p < .001, and ****p < .0001 were considered significant.
AUTHOR CONTRIBUTIONS
Tianle Tang: Conceptualization; data curation; formal analysis; visualization; methodology; software; and writing—original draft. Jinyan Chen: Conceptualization; data curation; project administration; validation; and writing—original draft. Jun Zhang: Conceptualization; data curation; project administration; validation. Pengcheng Pan: Project administration and resources. Jiahui Jiang: Project administration and resources. Chenyu Hu: Project administration and resources. Yuan He: Data curation. Chenchen Li: Conceptualization; validation; formal analysis; and supervision. Junfeng Zhang: Conceptualization; formal analysis; resources; software; and writing—review and editing. Yanli Wang: Conceptualization; funding acquisition; formal analysis; resources; software; and writing—review and editing.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (82325030, 52371250, and 42067040), Science and Technology special fund of Hainan Province (ZDYF2024SHFZ080), National Key Research and Development Program of China (2022YFC2305000), and ‘Nanhai Xinxing’ Science and Technology Innovation Talent Platform Project of Hainan Province (NHXXRCXM202318).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Research data are not shared.