Engineering multifunctional adhesive hydrogel patches for biomedical applications
Abstract
Traditional patches, such as sticking plaster or acrylic adhesives used for over a hundred years, lack functionality. To address this issue of poor functionality, adhesive hydrogel patches have emerged as an efficient bioactive multifunctional alternative. Hydrogels are three-dimensional, water-swellable, and polymeric materials closely resembling the native tissue architecture. The physicochemical properties of hydrogels can be modified easily, allowing them to be suitable for various biomedical applications. Moreover, adhesive properties can be imparted to hydrogels through physicochemical manipulations, making them ideal candidates for supplementing or replacing traditional sticking plaster. As a result, sticky hydrogel patches are widely used for transdermal drug delivery and have even found commercial purposes. Beyond transdermal delivery, such hydrogel patches have also found applications in cardiac therapy, cancer research, and biosensing, among other applications. In this mini-review, we critically discuss the challenges of fabricating multifunctional adhesive hydrogel patches. Furthermore, we introduce some of the chemical strategies involved with fabricating the patches. We also review their emerging biomedical applications. Finally, we explore their potential future in the flourishing field of tissue engineering and drug delivery.
1 INTRODUCTION
Traditional medical patches are routinely used for various biomedical applications. The most common patches include sticking plaster (more commonly referred to as “Band-aid®”) and acrylic tapes.1 Although effective to a certain extent, these patches severely lack biological functionalities. More recently, such traditional patches have been supplemented with electrodes for diagnosing ailments using echocardiograms, electroencephalograms, or electromyograms.2 However, the next generation of adhesive patches is being developed with integrated biological functionalities that can not only render but also substantially enhance the therapeutic and diagnostic outcome. Bioactive multifunctional adhesive patches (i) can deliver drugs by contact-free triggering with external stimuli, (ii) have inherent antimicrobial properties, and (iii) have superior detection abilities for diagnosing ailments. For instance, Jiang et al. designed a skin-friendly adhesive patch for treating chronic wounds.3 In the case of such chronic wounds, inflammation persists, which in turn inhibits the normal process of healing. The designed patch showed excellent anti-inflammatory properties, accelerating the process of healing. More adhesive patches have emerged that treat chronic wounds and promote tissue regeneration.4, 5 These next generations of medical adhesive patches are fabricated with three-dimensional, crosslinked polymers called hydrogels and are finding applications in transdermal wound healing, cardiac therapy, bone regeneration, and electrochemical sensing.
Adhesive hydrogel patches are viscoelastic scaffolds, have a large liquid-to-polymer mass ratio, and can closely resemble the native tissue constructs.6 The high-water retaining capacity is especially critical for wound healing-based applications where large volumes of wound exudate need to be absorbed.7 The constituent polymers of the hydrogels can be either synthetic or natural and are biocompatible, biodegradable, and easily tunable physicochemical properties.8, 9 Different polymer crosslinking strategies have been employed for preparing mechanically tough hydrogels suitable for fabricating adhesive hydrogel patches. These crosslinking strategies include covalent bonds,10 ionic-covalent bonds,11 and dynamic hydrogen bonds,12 among others.13 Beyond chemical crosslinking strategies, tough hydrogel patches can also be fabricated by integrating multiple polymers in the network, such as interpenetrating network14 and double network hydrogels.15 Additionally, mechanically tough hydrogels can be prepared by reinforcing the polymeric network using nanoparticles.16
Reinforcing hydrogel patches with nanoparticles also incorporates multiple material-based functionalities. Nanoparticles can be responsive to external stimuli, such as light energy,17 ultrasound energy,18 change in pH,19 magnetic field,20 and electric field.21 These properties can then be exploited to prepare state-of-the-art multifunctional adhesive hydrogel patches suitable for biomedical applications. Recently, Li et al. designed one such multifunctional hydrogel patch for healing skull defects.22 Here, β-cyclodextrin reduced graphene oxide was used to functionalize a gelatin-based hydrogel and mimic the electroconductive environment of the native tissue. Reduced graphene oxide increased the mechanical stability of the material, improved the conductivity, and promoted osteogenic differentiation. In another instance, Yang et al. fabricated cellulose nanocrystal functionalized hydrogel patch-based ionic sensor.23 The nanocrystal was used to improve the mechanical strength and stretchability of the polymer. Such unique material properties can be further harnessed to prepare the next generation of adhesive hydrogel patches for solving biomedical issues. Figure 1A highlights the different properties of sticky hydrogel patches and their potential biomedical applications. On the other hand, Figures 1B, C demonstrate the trend in the application of such hydrogel patches. Herein, we will review the physicochemical strategies employed for making adhesive hydrogels, evaluate the different hydrogel fabrication techniques, discuss their latest applications in biomedicine, and provide an insight into their prospects.
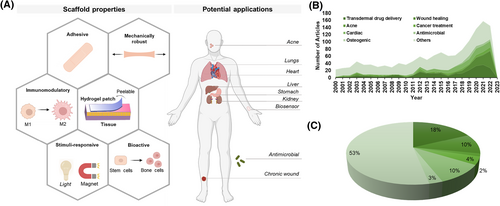
Hydrogel patches for regenerative applications. (A) Illustration outlines the different hydrogel patch properties and their biomedical applications. (B) Demographics highlight the increasing trend in the use of hydrogel patches in the last two decades. The database of Web of Science was used to generate the figure. Each term (e.g., transdermal drug delivery) was paired with “hydrogel patch” to determine the articles published in that category. (C) The pie chart displays the distribution of application using hydrogel patches between 2000 and 2023.
2 PHYSICOCHEMICAL STRATEGIES FOR HYDROGEL PATCH ADHESION TO WET SURFACES
The physiological environment of tissue surfaces is moist and wet because of various biological fluids, such as blood, interstitial, and excreted fluids, among others.24 As a result, hydrogel patches must be designed adequately to achieve sustained adhesion to wet surfaces and facilitate tissue repair and regeneration. However, even the most adhesive hydrogels exhibit poor adhesion to wet surfaces.25 Advanced chemical strategies need to be developed to improve their adhesive strength. These chemical strategies include covalent bonding, non-covalent bonding, or a combination of both.24, 26 One method for covalent adhesion can be achieved by adding specific functional groups to the adherend and hydrogel surfaces. By chemically modifying surfaces to covalently bond to the hydrogel, high strength bonding can be achieved, where functional groups on the polymer chains are chemically reacted with groups on the target adherend.27 Effective covalent bonds can be fabricated by understanding which functional groups are present at the surface of the adherend and hydrogel. For example, H. Yuk et al. demonstrated that high interfacial adhesion could be achieved by chemically modifying surfaces to bond to the polymer network in a tough hydrogel covalently.28 Since the major constituents of hydrogels are long polymer chains, this bonding was achieved by covalently crosslinking the long chain network to silanes on the modified adherend surface.
An alternate mechanism to create strong adhesion between hydrogel patches is through non-covalent bonding. This includes bond types such as hydrogen bonds, ionic interactions, and Van der Waals forces. One example of this type of bonding can be seen in a DNA-inspired, tough, wet-adhesion hydrogel prepared via radical polymerization of hydrophilic acrylic acid, acrylated adenine and uracil nucleotides, and hydrophobic methoxyethyl acrylate.25 Here, the robust mechanism of adhesion is attributed to the non-covalent, hydrogen bonds from the adenine and uracil molecules, as well as hydrophobic interaction from the methoxyethyl acrylate. Additionally, L. Han et al. developed a tough polydopamine–polyacrylamide hydrogel via π–π stacking and hydrogen bonds.29 This non-covalent bonding hydrogel has excellent tissue adhesiveness, as well as self-healing ability. During hydrogel synthesis, the catechol groups of the polydopamine chains were crossed-linked to the amino groups of the polyacrylamide network. This allowed for uniformly diffused non-covalent bonds to form between the catechol groups of the polydopamine chains. Furthermore, the free catechol groups from polydopamine interact with amine or thiol groups on various surfaces responsible for strong adhesion by cation–π or π–π interactions. Therefore, these non-covalent bonds contributed to the toughness and adhesivity of the hydrogel.
Strong hydrogel patch adhesion may also result from a combination of covalent and non-covalent bonds. For example, catechol groups in paired lysine-catechol-functionalized hydrogels bind to surfaces in various ways, including hydrophobic interactions on polymeric surfaces and coordination bonding to mineral and oxide surfaces.30 When adhering to hydrophobic surfaces, lysine evicts hydrated cations from the mineral surface, and the catechol can orient itself to bond to underlying oxides. Figure 2A displays one such chemical strategy, where both covalent and non-covalent forces were used for adhering the patch to wet surfaces.31 Figures 2B–D show the adhesivity of the designed patch to various organs. Future considerations for developing strong adhesive hydrogels could include synthesizing hydrogels with a higher composition of polymer network and molecules capable of creating strong covalent bonds with the adherend surfaces.
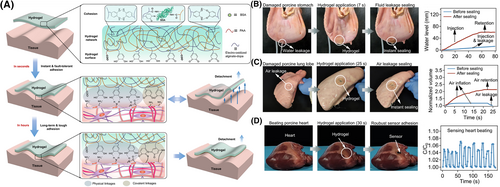
Chemical strategy for hydrogel patch adhesion to wet surfaces. (A) Schematic displays the chemical strategy implemented for adhering the designed hydrogel patch to wet surfaces. Here, alginate-dihydroxyl phenylalanine (dopa), bovine serum albumin, and polyacrylic acid polymers were used to prepare the tough hydrogel patch. Bovine serum albumin was covalently crosslinked to polyacrylic acid or alginate using the 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC)- N-hydroxy succinimide (NHS) coupling reaction. Moreover, hydrogen bonds formed between alginate-dopa, amine groups, and carboxylic groups. Initially, the hydrogel was able to stick to wet tissue. Additionally, over longer periods of time, dopa oxidized to dopaquinone to form highly adhesive hydrogel patches. (B) The designed hydrogel patch was used to seal water-leaking porcine stomach. The corresponding graph shows an increase in the water level in the stomach post-sealing. (C) Figure displays an air-leaking porcine lung. The developed patch was used to seal this leak, and the corresponding graph suggests an increase in lung air volume after applying the patch. (D) A capacitive strain sensor was stuck to a porcine heart using the designed hydrogel to sense the beating process. An air pump simulated the beating mechanism of the heart, and the corresponding graph displays healthy beating. Reproduced with permission.31 Copyright 2021, Springer Nature.
Finally, the physical properties of hydrogels also play a significant part in determining their adhesive behavior. The elasticity of the hydrogel is one such property that requires careful attention. Recently, Lee et al. fabricated an adhesive hydrogel patch where the elasticity of the material was modulated by exploiting the crosslinking mechanism.32 Besides elasticity, the mechanical strength of the patch is another critical parameter that needs to be analyzed to synthesize adhesive patches. A robust mechanical strength helps in maintaining the structural integrity of the patch. Adding inorganic fillers, such as nanoparticles, to the hydrogels may increase their mechanical strength.16 The addition of another polymeric network can also increase the mechanical strength of the hydrogels.33 However, higher mechanical strength also increases cohesive forces, leading to poor adhesion.34 Ideal adhesive hydrogel patches require a fine balance between tissue adhesive forces and polymer cohesive forces. If higher mechanical strength is desired, adhesive forces should be increased proportionately by chemical manipulation. Overall, understanding physicochemical properties is the key to designing sticky hydrogel patches capable of adhering to wet tissue surfaces.
3 ADVANCED HYDROGEL FABRICATION TECHNIQUES
In this section, we will review some state-of-the-art techniques that are implemented in synthesizing mechanically robust and stable hydrogels that can serve as a platform for preparing adhesive patches. By using physicochemical crosslinking strategies outlined in Section 2, these hydrogels can adhere to wet tissue surfaces. Chemical approaches in the form of Michael addition, Schiff base reaction, host-guest interaction, and coupling reaction, among others can contribute to creating tissue adhesive hydrogel patches. Specifically, in this section, we will discuss three-dimensional (3D) bioprinting, preparing microneedles, and cryogelation that have been used recently for developing adhesive hydrogel patches. Ultimately, innovation in these fabrication techniques will lead to future clinical translation.
3.1 3D bioprinting
3D bioprinting is a computer-aided, layer-by-layer additive manufacturing technique to artificially construct living and non-living scaffolds comprising organic materials, inorganic materials, stem cells, somatic cells, or any combination of the above.35-37 3D bioprinting has become a frequently used scaffold fabrication technique because of its strong ability to control the structure of the scaffold.38 Unlike other methods, 3D bioprinting can selectively place cells within the scaffold.39 Additionally, it has proven to be very cost-effective and scalable compared to other fabrication methods. Printing parameters (such as temperature, viscosity, and nozzle diameter, among others) and crosslinking strategies of the bioinks strongly influence the efficacy of the end product.40 Despite the advantages mentioned above, 3D bioprinting still contains many challenges, such as their limitation in selecting printing bioinks. High-viscosity bioinks tend to obstruct the extruder and impede the printing process.41 Another challenge associated with 3D bioprinting is maintaining a careful balance between the rheological properties of the pre-gel solution and the mechanical toughness of the final printed structure.42 Despite the challenges and disadvantages discussed here, recently, adhesive hydrogel patches have shown great potential in tissue engineering and drug delivery.43-47 Melhem et al. designed a 3D printed hydrogel patch using poly (ethylene glycol) dimethacrylate for treating myocardial infarction.48 Excellent tissue adhesion was achieved by applying fibrin glue to the developed patch. Thus, a two-step process was implemented in preparing this adhesive hydrogel patch, where the hydrogel was fabricated first, and the glue was added next to impart the adhesive behavior. Together with its ability to align living cells in desired orientations and patterns, 3D bioprinting is a prominent fabrication technique to construct desirable hydrogel patches for personalized therapy.
3.2 Microneedles
Microneedle systems are minimally invasive and painless transdermal drug delivery devices that can be accommodated into adhesive hydrogel patches.49-52 Hydrogel patches have been successfully combined with microneedle systems for effective drug delivery.53 When designing such a hydrogel-forming microneedle, the length of the needles is an important parameter.54 For a delivery system to be considered minimally invasive and painless, the length must not reach our pain receptors deep in the dermis of the skin, making shorter needle length, 500–800 μm desirable.55 Beneficially, as the needle length decreases, the mechanical strength increases.56 Additionally, the diameter of the tip has a great impact on skin penetration. It has been found that a tip diameter below 15 μm yields the best results.16, 57 Recently, Han et al. devised an exosome delivery vehicle as a hydrogel microneedle array patch for treating spinal cord injury.58 The patch was fabricated with biocompatible, biodegradable, and cell-adhering gelatin methacryloyl biopolymer,59 making the patch suitable for future translation. Although promising, microneedle-based hydrogel patches are yet to reach the commercial market, and further research initiatives need to be undertaken for the rapid evolution of this fabrication scheme. For example, one of the key challenges with microneedle hydrogel patches is the requirement of a liquid drug reservoir, which can be detrimental to the drug when subjected to high temperatures.60 Additionally, there is a lack of controlled drug release from the microneedle hydrogel patches as the microneedles biodegrade in the skin at uncontrolled rates, limiting the effectiveness of sustained drug delivery.61 Therefore, it is necessary to enhance the stability of the drug within the patches and develop different methods of releasing drugs in future research to produce a viable drug delivery system with microneedles.
3.3 Cryogelation
Cryogelation is a fabrication technique that can produce macroporous hydrogel patches with a high swelling ratio, increased drug loading capability, and enhanced cellular mobility.62, 63 Cryogelation refers to gels forming under sub-zero conditions, which results in a cross-linked polymer network surrounding ice crystals.64, 65 After removing the gel from the sub-zero environment, the ice crystals melt and leave a macroporous, interconnected structure, mimicking the extracellular matrix. These gels are called cryogels and have recently gained attention as tough, stable patches for biomedical applications.66-69 With large pore sizes, these patches usually demonstrate high drug-loading capacity. The fabrication of such porous and tough hydrogel patches holds a promising future in drug delivery. Recently, Rana et al. fabricated a gelatin-based “mussel-inspired” adhesive cryogel by incorporating acrylated dopamine into the hydrogel.70 Similar strategies can be implemented to prepare cryogel-based adhesive patches for biomedical applications.71, 72 However, cryogelation has several disadvantages. First, hydrogel prepared with the physical cross-linked cryogelation method has been reported with reduced physical properties such as unreliable degradability of hydrogel and inadequate gel integrity. These cryogels were too brittle and rigid compared to hydrogel prepared with other methods, as the crosslinking is weak and unstable.73 Second, the hydrogel synthesizing process of the cryogelation method involves gelation, freezing, and thawing, which is both time-consuming and energy-consuming, making the whole process inefficient and unfavorable for mass production. Finally, hydrogel patches synthesized from cryogelation methods result in uneven pore sizes and shapes, which may affect the degradation rate of the hydrogel, and affect the drug release rate.74 Further research is needed to improve this fabrication strategy and prepare state-of-the-art tissue adhesive patches. Table 1 highlights the different fabrication schemes used for preparing hydrogel patches intended for drug delivery. In the following section, we will discuss the use of nanoparticles for functionalizing hydrogel patches.
| Fabrication strategy | Polymer | Crosslinking chemistry | Mechanical strength | Therapeutic molecule | Application | Ref |
|---|---|---|---|---|---|---|
| 3D bioprinting | Gelatin methacryloyl | Photo-crosslinked using ultraviolet (UV) light—covalent bond | - | QHREDGS peptide | Skin wound healing | 46 |
| Hyaluronic acid methacryloyl | Angiogenesis | |||||
| 3D bioprinting | Carboxymethyl chitosan | Carboxyl group from oxidized polysaccharide crosslinked with amine group from carboxymethyl chitosan—covalent bond | Stiffness: 55–426 Pa | - | Scleredema diabeticorum | 47 |
| Oxidized polysaccharide (snake gourd root/Astragalus = 1/3, w/w) | ||||||
| Microneedles | Methacrylated hyaluronic acid | Photo-crosslinked using ultraviolet (UV) light—covalent bond | Each needle withstands 0.3 N | - | Extraction of skin interstitial fluid | 51 |
| Microneedles | Polyvinylpyrrolidone | - | - | Triptolide | Rheumatoid arthritis | 52 |
| Cryogelation | Hydroxypropyl methacrylamide | Dialdehyde starch crosslink—covalent bond | - | Quaternary poly (4-vinyl pyridine) | Wound dressing | 68 |
| Glutaraldehyde crosslinked cryogel to quaternary poly (4-vinyl pyridine)—covalent bond | Vitamin C | |||||
| Cryogelation | poly (vinyl alcohol) | Hydrogen bonds | Tensile strength: 1.3 MPa | - | Abdominal wall defect treatment | 69 |
| Compressive strength: 2.1 MPa |
4 NANOPARTICLE-FUNCTIONALIZED ADHESIVE HYDROGEL PATCHES
Hydrogel patches are commonly used as drug delivery systems, but one of their main limitations is their rapid/burst release.75 Moreover, natural biomaterial-derived polymers lack mechanical strength and need reinforcement for their use as adhesive patches. The incorporation of nanoparticles helps in addressing both these concerns by controlling the burst and sustaining the release of therapeutic molecules as well as serving as mechanical reinforcing agents.16, 76, 77 Nanoparticles improve mechanical strength by increasing the hydrogel's resistance to deformation during knotting, tearing, twisting, and bending.78-80 Nanomaterials can also impart structural color to hydrogel patches (Figure 3).81 Figure 3A highlights the mechanism of adhesion, which uses both covalent and physical bonds. Figure 3B demonstrates the superior toughness of the designed material when compared to commercially available patches. Figure 3C displays the fabrication strategy of adhesive hydrogel patches with vivid colors that resulted from the incorporation of silica nanoparticles. Figure 3D demonstrates that the silica nanoparticles can improve the tensile strength and strain of the hydrogel patch system significantly. Finally, Figure 3E shows the viability of the designed product. Additionally, nanomaterials within hydrogels can improve cell and protein adhesion due to their large surface area to volume ratio leading to more adsorption.82 Nanoparticles can also incorporate unique physicochemical, mechanical, and biological functionalities into hydrogel patches. Such nanomaterials can make the patches responsive to external stimuli, such as heat,83 light,84 pH changes,85 and electromagnetic field.86, 87
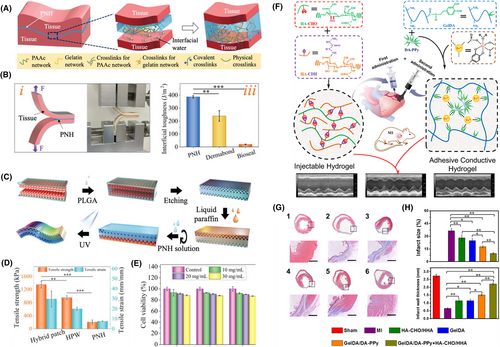
Nanoparticle-incorporated wet adhesive hydrogel patches. (A) Schematic illustrates the mechanism of double-sided hydrogel adhesion to wet surfaces. The hydrogel patch (labeled PNH) consists of polyacrylic acid (PAAc), acrylate (polyethylene glycol) N-hydroxisuccinimide (aa-PEG-NHS), and gelatin. Strong tissue adhesion was achieved by both covalent and physical bonds. (B) The interfacial toughness of the designed hydrogel was tested by a 180° peel test and compared to commercially available tissue adhesives. (C) Illustration outlines the fabrication scheme of an even superior adhesive hydrogel patch that displays dual surface properties. Here, colloidal crystal templates were prepared using silica nanoparticles. Poly lactic co-glycolic acid (PLGA) was used as the primary scaffold, and the void spaces in the crystal template were filled with PLGA. An inverse opal structure was achieved by immersing the template in hydrogen fluoride, etching the silica surfaces. Liquid paraffin was used to inhibit the adhesiveness of one of the sides, making the hydrogel less harsh to the targeted tissue. The previously designed pregel was added at this point to prepare the final adhesive patch. (D) Graph demonstrates the superior mechanical strength of the final patch compared to that without the inverse opal design (HPW) or the previously formed hydrogel. (E) Chart displays the fibroblast viability of the designed adhesive patch. Reproduced with permission.81 Copyright 2022, Wiley-VCH GmbH. (F) Schematic shows an adhesive hydrogel patch co-administered with injectable hydrogel for treating myocardial infarction. The adhesivity of the hydrogel patch was achieved by using dopamine-modified polymers, gelatin (GelDA) and polypyrrole (DA-PPy) nanorods. Polypyrrole enhanced the electroconductivity of the designed patch. (G) Masson trichome stains display a reduction in fibrosis with the hydrogel treatment. (H) Graphs show a reduction in infarct size and an increase in the left ventricular wall thickness after hydrogel patch treatment. Reproduced with permission.90 Copyright 2019, American Chemical Society.
Stimuli-responsive nanocomposite hydrogel patches are frequently finding applications in tissue engineering. Stimuli-responsiveness imparts multifunctionality to the developed patches. For instance, converting light to thermal energy can help in tumor ablation, thus broadening the applicability of the patches. Near-infrared light is often used to stimulate hydrogels and increase their temperature.17 Matai et al. have developed a hydrogel patch with photothermal heating capabilities and on-demand drug release using polyvinylpyrrolidone-functionalized graphene oxide nanosheets and trimethylammonium bromide gold nanorods.88 The patch shows promise in targeting, shrinking, and preventing tumor recurrence in localized tumors. Nanoparticles can also incorporate electrical conductivity, which can lead to the fabrication of excellent cardiac patches where electroconductivity is critical. For instance, Hosoyama et al. have fabricated a collagen-based hybrid nanoengineered electroconductive patch for cardiac regeneration.89 The nanogold-incorporated patches developed here were able to restore cardiac function without relocating to neighboring organs. Figures 3F–H show another nanoparticle-reinforced adhesive hydrogel that can be used for treating myocardial infarction.90 Here, polypyrrole nanorods were used to impart conductivity to the hydrogel patch. Furthermore, using magnetic nanomaterials and applying an external magnetic field can also manipulate the rate of drug release from the designed hydrogels.91 Increased efforts in developing such multifunctional nanocomposite hydrogels will lead to the rapid evolution of this technology in biomedicine.
5 ELECTROCHEMICAL SENSING WITH ADHESIVE HYDROGEL PATCHES
An electrochemical sensor is a device that can provide continuous monitoring and measurement of a desired analyte. Advances in electrochemical sensing platforms have helped develop personal monitoring devices that can sense physiological information and help detect underlying health conditions.92-95 Specifically, hydrogel-based electrochemical sensors fit a unique need in the market that provides a method for cost-effective, convenient, and continuous measurement. By using hydrogel-based sensors, biofluids such as tears, saliva, or sweat can be used as a sampling target rather than blood, making these sensors a non-invasive, convenient approach for providing information about a patient.96 For biomedical applications, these sensors can be extremely valuable in detecting physiological analytes that can reflect underlying health conditions such as diabetes,96 dehydration,92 flatfoot,94 chronic psychological stress,92 or pain.93
In terms of the non-invasive detection of physiological conditions, there are various methods for sensing, including sweat rate92, 93 or composition analysis,93, 96 intestinal disease detection,97 as well as strain sensing.94 Hydrogel patches aid in detecting sweat volume loss or sweat rate by wicking of sweat into hydrogels and measuring the swelling of their physical geometry.92 Here, the hydrogels are made super-absorbent by using polyvinyl alcohol, which allows for the quick uptake of sweat into the sensor patch. The geometry change of the swelling hydrogels can be monitored by measuring the area of the patch through a smart-phone or by optical reflectance that can be continuously monitored using a smart-watch. This detection and measurement provide the basis for calculating the sweat loss rate of the individual. The ultra-simple patch design allows for the sensing of sweat volume loss while only using adhesives, textiles, hydrogels, and clear cover materials.
For the compositional analysis of detected sweat, electrochemical electrodes can be incorporated into the sensor. For external sensing using hydrogel patches, the patch is attached using an adhesive on the skin in an accessible location such as the finger, palm, or back of the hand.93, 96 When the patch adheres to the skin, the continuously hydrophilic pathway created by the polyvinyl alcohol-coated agarose-glycerol hydrogel patch allows sampling metabolites in the sweat present on the skin.96 For using electrochemical electrodes in these sensors, two reference electrodes, and two ion-selective electrodes for a desired analyte are needed. The electrochemical sensors functionalized into the patch can detect different aspects of sweat composition, such as pH, chloride ions, glucose, or drug concentration, by measuring the potential difference between the ion-selective electrodes and the reference electrode. For example, the pH-selective electrode's potential changes with pH due to deprotonation of the electrode's conductive polyaniline film, whereas the chloride ion-selective electrode changes the redox equilibrium between an Ag/AgCl electrode to create a measurable change in the sensor's potential signal.93 Once collected by the sensor, sweat composition data was collected using an electrochemical workstation for analysis. Patients can apply such hydrogel patch-based sensors to continuously monitor their physiological status and health condition for enhanced therapy.
Finally, compression sensing properties of conductive hydrogels can be used for the preliminary detection of human diseases such as flatfoot.94, 98 Flatfoot is an acquired developmental disease that can lead to complications such as arthritis or disability in adulthood.99 Therefore, conductive hydrogel-based sensors can be made into plantar pressure sensors so that pressure signals can be stably detected, providing a novel strategy for the diagnosis and correction of flatfeet. A hydrogel sensor used for detection was prepared using dialdehyde carboxymethyl cellulose, amino gelatin, and polyacrylic acid, which had excellent adhesion to skin, ductility, biocompatibility, and electrical conductivity to ensure the stability of electrical signal output from the sensor.94 The conductive hydrogel was adhered to a copper sheet, which was connected to an electrochemical workstation for a complete circuit. When encountered by an external force, such as the planar pressure from a patient, the conductive hydrogel sensor would deform, resulting in changes in resistance and current values.94 The detection of the electrical signal results indicates that the hydrogel sensor is an ideal candidate as a strain sensor to detect human plantar pressure stably.
In summary, electrochemical hydrogel-based sensors have enormous potential for health monitoring and detection. Not only are these sensors noninvasive for patient comfort, but these sensors are also flexible, cost-effective, convenient, easy to synthesize, and provide continuous and reliable measurement. Hydrogel-based sensor detection provides a novel method for personalized healthcare, clinical diagnosis, and physical monitoring and could play an integral role as future developments are made.
6 BIOMEDICAL APPLICATIONS OF ADHESIVE HYDROGEL PATCHES
Owing to their excellent (i) biocompatibility, (ii) biodegradability, (iii) cell modulating behavior, (iv) ability to load and sustain the release of entrapped therapeutics, and (v) response to external stimuli, hydrogel patches have gained much attention in the field of regenerative medicine. Here, we critically analyze some of the latest applications of hydrogel patches and discuss their therapeutic potential.
6.1 Transdermal drug delivery with hydrogel patches
The traditional drug delivery pathways are via oral administration or hypodermic injections, but these methods have their limitations.100 Transdermal drug delivery can overcome these limitations as it has painless delivery, sustained release potential, and patient ease of use, and it eliminates the risk of disease transmission and contamination as it is a needle-free approach.34, 101 The main limitation in transdermal drug delivery is uncontrolled drug delivery and skin permeability.102 Hydrogel patches can act as effective transdermal drug delivery systems and can help overcome these drawbacks, where the adhesive nature of the patches will hold the drug delivery system in place without the need of sutures or bandages. These patches can be formulated with tunable mechanical strength and strong skin adhesion properties. The following subsections will touch on hydrogel patches as transdermal drug delivery systems in applications such as wound healing, soft tissue injuries, and acne treatment.
6.1.1 Patches for wound healing
Wound healing is a complex process as it is a result of a variety of different factors that influence how the skin heals.103 Subsequently, if normal healing does not progress, a chronic wound may develop, resulting in undesired pain and high medical bills.103 Wounds are easily susceptible to harmful microorganisms and other foreign particles. Subsequently, immune cells are drawn to the damaged area to combat the foreign invasion leading to inflammation. In the case of chronic wounds, the inflammation period persists, prolonging the time needed for the wound to heal.104 It is reported that chronic wounds constitute over $25 billion of medical bills in the United States alone.105 Dressings, such as gauzes and bandages, can decrease the risk of infection as they provide a protective layer, but they are not able to induce wound healing or closure.106 Therefore, a vast amount of research is geared toward developing hydrogel patches that can promote wound healing and closure.107-113 The adhesive hydrogel patch can act both as a drug delivery platform to cargo drugs and facilitate wound healing and act as sterile dressing to keep the wound covered, providing a protective layer.
6.1.2 Patches for treating acne
Acne affects 85% of people aged 12–25 and can often continue into the earlier stages of adult life.114 It is an inflammatory disorder of the pilosebaceous unit and can negatively affect the patient's quality of life, as it is associated with a higher rate of anxiety and depression.115 Common treatments for acne are oral and topical antibiotics, including erythromycin, clindamycin, and tetracyclines.116, 117 Adhesive hydrogel patches can help to treat this disorder.116, 118, 119 Chitosan has unique characteristics for an ideal acne patch, including its antibacterial properties, biodegradability, and biocompatibility.116 Similarly, gelatin is a hydrophilic polymer derived from denaturing collagen with better biocompatibility and biodegradability properties.120 Together, these polymers can formulate innovative hydrogel acne patches.116 Additionally, researchers are working on chitosan nanoparticles loaded with nutraceutical nicotinamide for topical acne treatment with promising clinical results.121 The integration of nanoparticles encapsulated in hydrogel patches could yield promising results and should continue to be explored moving forward.
6.2 Cardiac therapy with hydrogel patches
Hydrogel patches are being investigated for their ability to treat myocardial infarction, commonly known as a heart attack. Following myocardial infarction, 30% of patients suffer from heart failure, a leading cause of death worldwide.122, 123 Although heart failure can be treated through medications, heart valve repair or replacement is not uncommon. Current preclinical studies have explored treatment methods for myocardial infarction by injecting various growth factors, stem cells, and genes.124 Over time, many limitations of injection-based drug delivery treatment for myocardial infarction became evident, including poor stability, short half-lives, and the inability to restore cardiac functionality.124, 125 However, cardiac patches have shown immense promise in recent clinical and preclinical studies treating myocardial infarction.89 These patches are typically composed of both substrates and therapeutic ingredients, including pluripotent stem cells, mesenchymal stem cells, and growth factors.124 Research has revealed that collagen-based cardiac patches have proven to restore and promote cardiac recovery.89 However, these collagen patches lacked the electrically conductive property in myocardial tissues. Therefore, these patches are unable to restore the conductive tissue that was damaged during myocardial infarction. To overcome this, a group of researchers created collagen-coated nanogold patches with excellent biocompatible and conductive properties.89 These hybrid patches were designed with a unique structure that included an electroconductive component within the collagen fibers while utilizing an elastic hydrogel for mechanical stability. When tested, the gold hydrogel patches demonstrated improved stability over three days relative to the pure collagen patches. Figure 4A highlights another strategy for preparing hydrogel patches for cardiac therapy.126 Here, a dual-layered patch was prepared, where the bottom layer served the purpose of adhesion to wet surfaces. Caffeic acid provided the necessary catechol groups for facilitating wet tissue adhesion. Figures 4B–E demonstrate the effectiveness of the designed patch.
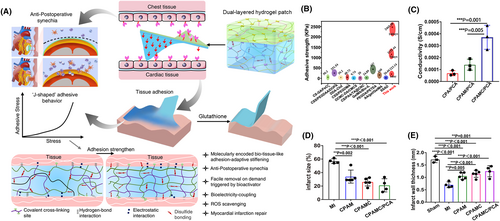
Cardiac therapy using hydrogel patch. (A) The illustration displays the adhesion of the hydrogel to wet surfaces. Here, the hydrogel comprised of interpenetrating networks of polyacrylic acid, polyethyleneimine, and aldehyde cellulose crosslinked using N, N′-bis(acryloyl) cystamine. The resulting covalent and non-covalent interactions of the interpenetrating network led to strong adhesion to wet surfaces. This adhesive region of the hydrogel formed the bottom layer, whereas carboxylated cellulose and acrylic acid formed the top layer. Additionally, the patch can be removed by using glutathione, which is able to break the disulfide bonds of the crosslinker molecule. (B) Graph displays superior adhesive strength of the designed patch when compared to that of materials obtained from the literature. (C) The designed hydrogel patch showed superior conductivity. (D) In vivo models display smallest infarct size in the case of the developed patch. (E) Data shows the thickest left ventricular wall in case of the treatment group. Reproduced with permission.126 Copyright 2022, Springer Nature.
Other than myocardial infarction, congenital heart defects are also a current biomedical concern. On average, congenital heart defects affect one in every 125 children that are born.127 Latest treatment methods include frequent catheterization, long-term medication, and stents.128 However, each of these methods are short-term solutions that have proven to increase the risk for other cardiovascular diseases. Curative treatment requires a surgical repair using either a polymer or a fixed tissue patch.127 Unfortunately, these types of patches are not compatible with pediatric patients, are not electromechanically integrated, and have repeatedly led to the formation of arrhythmias and even cardiac arrest and death. In a recent study, a hydrogel patch has been prepared for treating congenital heart defects. The patch is composed of polyethylene glycol reinforced with an electrospun biodegradable poly (ether ester urethane) urea mesh.127 In vivo results show that after 8 weeks post-implantation, the hydrogel patch resulted in reduced fibrosis and an increase in infiltration of endothelial cells in comparison to fixed pericardium patches. This was in addition to improved muscular and vascular ingrowth and reduced unwanted immune responses. However, an increased effort in this area is needed for clinical translation.
6.3 Combating microbial infection with hydrogel patches
In the past decade, the need to manage microbial infections has drastically increased.129 This results from the continuous growth seen in the emergence of resistant bacteria posing a public health problem.130 Most acute global mortality in the US is caused by infectious diseases.130 Clinicians have found that treating patients using wound dressings, joint implants, and catheters has significantly increased the number of implant-associated infections.130 As a result of the misuse of drugs and antibiotics used to treat these infections, a surge in drug-resistant bacteria has resulted.130 At present, there is an urgent need for antimicrobial biomaterials and medical devices that are capable of local and sustained delivery of drugs, which minimizes their use. In order for local applications to be successful, the drug delivery system should not only have antimicrobial effects but also be biodegradable.130 For this reason, researchers have explored the synthesis of antimicrobial hydrogel patches.129-131
Hydrogels have proven to be highly biocompatible and can foster an environment that supports tissue repair and regeneration.131 They can be synthesized using biomedical polymers such as chitosan, which are inherently antimicrobial in nature. Additionally, hydrogels can be loaded with antimicrobial substances such as silver ions and antibiotics.129-131 Researchers have discovered that antimicrobial hydrogel patches can be applied to catheter-associated infections, contact lenses, and urinary tract coatings.130 Through various studies, it was found that effective antimicrobial hydrogel patches could be synthesized through various methods, including photopolymerization, cryogelation, and freeze-thawing.63, 129, 131 Du et al. explored a multifunctional hydrogel patch that was not only antimicrobial but also served as a means for sutureless wound closure.132 The patch was composed of poly (ethylene glycol) diacrylate, quaternized chitosan, and tannic acid and exhibited strong mechanical properties, low swelling, and high antimicrobial activities against E.coli and S. aureus. Here, tannic acid derived from natural sources and containing high quantities of catechol groups helped adhere the patch to tissue surfaces. As a result of the positive antimicrobial properties of these hydrogels alongside their superior mechanical properties, these trials have proven that multifunctional antimicrobial hydrogel patches can play a large role in the biomedical field.
6.4 Bone and cartilage regeneration with hydrogel patches
Typically, hydrogels are used as a drug delivery system for the regeneration and repair of tissues.133 However, with the increase in medical costs relating to the treatment of bone trauma, repair, and infections, the need for bone regeneration treatment is crucial.134 Current treatment methods include allografts, autografts, and xenografts, each bearing the risk of infection, rejection, and disease transmission. A treatment approach incorporating bioactive growth factors is thought to be an innovative treatment method to overcome the issues mentioned above. A group of researchers in Korea sought to develop highly osteoconductive hybrid hydrogel patches for the treatment of bone repair.135 The design of the patches included inorganic minerals, hydroxyapatite, or whitlockite encapsulated into pyrogallol-conjugated hyaluronic acid. These patches were applied to mice to analyze their ability to generate bone formation in vivo. It was determined that these patches were easily applied with no need for additional glues or crosslinking. Additionally, after 8 weeks, mice treated with the hybrid hydrogel patch presented with significantly smaller bone defects than mice that received no treatment. The positive results from this study highlight the versatility of hydrogel patches and their diverse applications in the biomedical field.
Osteogenic hydrogel patches were also examined for their ability to treat craniocerebral injuries effectively.136 These large skull defects are often difficult to treat and are life-threatening.136 A common treatment for such large injuries involves a two-step method that includes craniectomy and cranioplasty.136 Although successful, this two-step method is unable to preserve auto-bone fragments and exposes brain tissue. For this reason, a one-step method involving an osteogenic hydrogel patch is believed to be an effective treatment strategy. A group of researchers in China developed a one-step elastic auto-bone patch designed with auto-bone fragments and a gelatin methacrylate hydrogel.136 Highly desirable tissue adhesion resulted from the use of methacrylated gelatin and N-(2-aminoethyl)−4-(4-(hydroxymethyl)−2-methoxy-5-nitrosophenoxy) butanamide (NB) linked to glycosaminoglycan hyaluronic acid (HA-NB). The incorporation of auto-bone fragments was found to accelerate bone healing and effectively regenerate skull defects. Furthermore, these patches did not induce an immunoreaction and protected the brain tissue from further injuries. Future directions for osteogenic hydrogel patches include the incorporation of nanoparticles.
Articular cartilage defects have also shown limited self-healing properties and remain difficult to treat.137, 138 Cartilage damage is a leading cause of reoccurring joint pain and disabilities in millions of individuals around the world.138-140 In fact, in the United States alone, 6% of individuals over the age of 30 have suffered from cartilage damage.138 Current treatment methods include the transplantation of cartilage plugs, subchondral microfracture, and autologous chondrocyte implantation.138 However, these methods are unable to restore the function of cartilage fully.137, 139 With the continual advances of hydrogels for biological applications, the use of hydrogel patches for cartilage regeneration is becoming a promising treatment method. For the replacement of articular cartilage, hydrogels can be used as cell carrier materials that encourage tissue regeneration or as implants to replace the damaged cartilage. The ability to incorporate stem cells using hydrogel patches is a unique approach to cartilage regeneration.137 Stem cells have shown chondrogenesis abilities, thereby restoring cartilage in an economic and facile manner. A group of researchers in Shanghai investigated the incorporation of stem cell-derived exosomes in a hydrogel tissue patch.137 To ensure the exosomes were securely embedded into the hydrogel patch, a photoinduced imine crosslinking glue was used. This glue was deemed suitable for cartilage regeneration as a result of its biocompatibility, adhesive properties, and strong operability.137, 141 The patches were then tested in vivo in a rabbit study where, after 12 weeks post-implantation, new tissue containing chondrocytes—cells found in healthy cartilage—covered the damaged cartilage. Additionally, it was clear that the hydrogel patch easily integrated with the native cartilage and successfully retained the exosomes. Future clinical studies are needed to confirm the patch’s effectiveness in humans; however, these positive results demonstrate promise for cartilage regeneration.
6.5 Ocular wound healing in large-size defects with new generation hydrogel patches
Among severe ocular injuries, open-globe injuries or full-thickness wounds compromise ocular protective barriers leading to severe damage to the integrity of the ocular surface and visual impairment.142 Large defects are also caused due to inflammation (auto-immune disorders) or surgical incisions from ocular surgeries (e.g., cataract surgery).143 Thus, these wounds pose great challenges for treatment since they need advanced surgical intervention (skills) and an operating theater. Given these challenges, surgical adhesives or patches have found applications for facilitating the closure of open-globe injuries, especially in emergency settings. Currently, the primary products used in the clinics are cyanoacrylate glues, fibrin glues, and polyethylene glycol-based sealants (e.g., ReSure® sealant). Their usage for closure of full-thickness wounds, however, is limited owing to the release of toxic by-products (cyanoacrylate glues), low adhesive strength (fibrin glues), and rapid degradation of material properties (ReSure®).144, 145 New generation hydrogel patches are thus in vogue to render not only strong adhesion properties to the tissue but also to provide the required mechanical/chemical/biological signals for tissue regeneration.
Jumelle and coworkers have developed a hydrogel patch based on three photocrosslinkable polymers, gelatin methacryloyl (GelMA), hyaluronic acid glycidyl methacrylate (HAGM), and PEG diacrylate (PEGDA).146 While GelMA provides excellent ocular tissue adhesion, HAGM possesses high viscosity, which enhances the retention of hydrogel prepolymer on the tissue surface before photopolymerization, and PEGDA renders flexibility, stretchability, and in vivo biodegradation of the patch. This patch can be applied beneath a contact lens and hence does not require any special surgical intervention. An ion-activated, dual-network hydrogel patch was reported by Zhao et al.147 Here, tetraceptides (Gly-Leu-Lys-Dopamine) were added to an alginate backbone to promote intramolecular bonding. Alongside, transglutaminases were used to stabilize the adhesive property of the material. They used natural corneal extracellular matrix and peptide-modified alginate. This composite material offered desirable transparency and biocompatibility, tunable mechanics, and robust adhesion. The presence of peptide in the hydrogel enabled the patch to maintain keratocyte phenotype in vitro. They reported that the designed patch rapidly sealed the large corneal defect and facilitated the formation of the normal curvature of the corneal tissue. This was comparable to the outcome of a donor corneal transplantation after 6 months. Another recent example is an electrospun patch functionalized with a reactive oxygen species (ROS)-scavenging hydrogel for treating ocular alkali burn injury, which is a serious ophthalmic emergency.148 Here, electrospun nanofibers of thioketal-containing polyurethana was integrated with a ROS-scavenging hydrogel containing thioketal diamine and 3,3′-dithiobis (propionohydrazide) crosslinked poly (poly (ethylene glycol) methyl ether methacrylate-co-glycidyl methacrylate). The patch demonstrated excellent transparency, high tensile strength, robust antioxidant activity, and reduced inflammation, resulting in accelerated corneal wound healing in a rat corneal alkali burn model.
A predictive modeling-based design of ocular hydrogel patches was reported recently by Gholizadeh et al.149 They performed a computerized multifactorial definitive screening design analysis and pinned down material properties that impact adhesion, swelling ratio, elastic modulus, and second-order interactions between applied components. A predictive model was developed that identified the linear and non-linear correlations between these parameters. Based on this, an optimized, photocrosslinkable adhesive patch based on two modified polymers, gelatin methacryloyl and glycidyl methacrylated hyaluronic acid, was developed. This patch demonstrated high burst pressure, minimal swelling, adhesion, and prolonged retention when applied ex vivo on the sclera.
Electroadhesion follows a unique adhesion mechanism, which is enabled by electrostatic interaction between the adherend and the adherent in an electric field.150 This mechanism has been harnessed recently in the context of ocular adhesives. Borden and coworkers have demonstrated electroadhesion between cationic gels and animal (bovine) tissues when placed under an electric field (DC, 10 V) for 20 s.151 The adhesion persists indefinitely and can be eliminated by applying the DC field with reversed polarity. They report that electroadhesion is effective against many tissues, including the aorta, cornea, lung, and cartilage. Electroadhered hydrogel patches provide a robust seal eliminating the need for sutures, and adhesion can be achieved on command, and in case of error, adhesion can be reversed.
6.6 Gastrointestinal tract healing using hydrogel patches
As the only organ system that provides usable fuel and building material such as water, electrolytes, lipids, minerals, protein, carbohydrates, and vitamins, irregularities developed within gastrointestinal tract can often lead to chronic diseases when ignored. Currently, there are many drugs that can treat gastrointestinal disorders and maintain a healthy gastrointestinal tract. Most of those drugs are at optimal efficacy when delivered orally, and oral delivery allows patients to self-administrate drugs without the need to seek medical professionals, reducing the strains and pressures on public health services.152 However, oral administration of most drugs results in a loss of their viability and bioactivity due to gastric acid and bile salts. To combat such issues, adhesive hydrogel patches can be used to encapsulate, protect, and release drugs inside the intestines while preventing the drugs from direct contact with gastric acid and bile salts. For instance, J. Lee et al. designed a catechol-conjugated adhesive hydrogel patch for treating gastrointestinal cancer.153 In addition to the wet tissue adhesive catechol-conjugated chitosan, the hydrogel also consists of a layer of polyvinyl alcohol intended to prevent mucus flow within the patch. Furthermore, the patch also contains magnetic nanoparticles and can be directed to the target site using an external magnetic field, thereby increasing the efficacy of the delivered therapeutic.
Recently, coating systems have also been developed to improve gastrointestinal epithelial function.154 These coating systems effectively deliver therapeutics and help in modulating nutrient, glucose, and water absorption and serve as a treatment strategy for metabolic disorders such as obesity, hyperinsulinemia, and diabetes.155 They can potentially replace invasive surgeries such as gastric bypass surgery and intestinal sleeve replacement. This coating system can also function as an intestinal barrier to prevent pathogenic invasions of the gastrointestinal tract.156 Additionally, hydrogel patches combined with wet-surface adhesiveness have the potency to replace or assist traditional sutures for repairing damaged and injured gastrointestinal organs.157 The porous structure of the hydrogel can greatly promote angiogenesis,158 and the organic ligament and polymer that forms the hydrogel patch can be readily absorbed or degraded in the body, avoiding the need for suture removal. Overall, the new generation multifunctional adhesive hydrogel patches have demonstrated promising preclinical outcomes, but clinical trials are warranted for tuning their in vivo performance and safety.
7 CONCLUSIONS AND FUTURE DIRECTIONS
In summary, recent research shows substantial progress in the preparation, characterization, and application of multifunctional adhesive hydrogel patches in biomedicine. Multifunctionalities in the form of external stimuli-responsiveness are added to these hydrogel patches to broaden their applicability. Such multifunctional materials can regenerate tissues, conduct electricity, display antimicrobial properties, act as biosensors, as well as deliver therapeutics in the presence of external stimuli. Although there are numerous studies on multifunctional hydrogels, their application as tissue adhesive patches is still relatively new. As a result, extensive research is still required to achieve commercial success.
The patches discussed here are excellent for the local delivery of therapeutics for treating chronic wounds.159, 160 Beyond local delivery at the wound sites, these sticky patches are excellent for delivering systemic transdermal medication because of their (i) biocompatibility, (ii) biodegradability, (iii) ability to load drugs, (iv) ability to sustain the release of the loaded drugs at a predetermined rate, and (v) ability to allow for cell attachment and infiltration. However, because of the barriers presented by the outermost layer of skin (epidermis), very few therapeutic molecules can be delivered to the bloodstream using skin-based simple polymeric hydrogel patches.161 This hydrophobic outer layer prevents the passive diffusion of therapeutics larger than 500 kDa.162 In this context, hydrogels can be supplemented with nanoparticles to overcome the barriers and increase the bioavailability of drugs. The nanoparticles can access the lymphatic and blood circulatory systems using dermal structures, such as sweat glands, hair follicles, nerve fibers, blood, and lymphatic vessels. Hydrogels can then serve as a reservoir for the drug-loaded nanoparticles, actively transporting the drug into the circulatory system.
Adhesive hydrogel patches have also emerged as a platform for transcutaneous immunization. Recently, Kamei et al. prepared a hydrogel patch for delivering antigenic proteins to activate memory cells critical for vaccination against cancer and infections.163 In another instance, hyaluronic acid-based transdermal hydrogel microneedles were prepared for treating skin cancer.164 Similar immunization platforms may be designed with nanoparticle-loaded hydrogel patches for advanced therapy. Other than being applied as an immunization platform, hydrogel patches have also found non-invasive applications in dental treatment. Favatela et al. designed a gelatin-based biocompatible patch for delivering anesthetic medication.165 These patches can provide an excellent alternative to traditional needle-based delivery systems, which can be uncomfortable or lead to unnecessary bleeding or even systemic complications. Additionally, hydrogel patches can also be used for immunomodulation necessary for regeneration, and Figures 5A–E show latest patches that have been developed for such purposes.166, 167 However, the applications of hydrogel patches are not limited to transdermal delivery routes. These wet tissue adhesive hydrogel patches are excellent for cardiac regeneration, electrochemical sensing, and ocular wound healing, among other biosensing and regenerative applications. Figure 6 demonstrates one such hydrogel patch that was designed for gastric repair.168
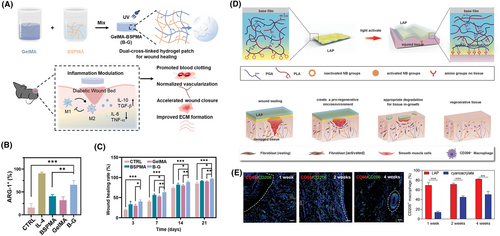
State-of-the-art immunomodulatory hydrogel patches. (A) Schematic represents a dual cross-linked immunomodulatory hydrogel patch. Here, methacrylated biopolymers (gelatin and Bletilla striata polysaccharide) were crosslinked using photo-initiator and UV light. The Bletilla striata polysaccharide has D-mannose in its polymeric chain, which can interact with the mannose receptors located on macrophages. This interaction leads to the transition of the macrophages from M1 phenotype to M2 phenotype, which is associated with pro-angiogenesis. With the help of this polymer, the devised hydrogel patch can transition M1 to M2 phenotypes, release pro-angiogenic factors (e.g., cytokines), and reduce the release of pro-inflammatory factors (TNF-α). (B) Arginase-1 (Arg-1) is a marker of macrophages with M2 phenotype. The immunofluorescence data suggest that the expression of M2 macrophages in the designed hydrogel patch group (B–G) was the highest. IL4 was added as a positive control. (C) In vivo studies show that full-thickness wounds heal faster with the designed B-G hydrogel. Here, the graph displays quantitative wound area at different times post-treatment. Reproduced with permission.166 Copyright 2022, Wiley-VCH GmbH. (D) (Top panel) The illustration depicts the design of a light-controlled adhesive hydrogel patch for effective immunomodulation and wound regeneration. The components of the hydrogel patch include γ-polyglutamic acid (PGA) bound to poly-l-lysine (PLA) as the hydrogel matrix and light active N-(2-aminoethyl)-4-(4-(hydroxymethyl)-2-methoxy-5-nitrosophenoxy) butanamide (NB) groups as the adhesive layer. Upon UV light irradiation, the Schiff base formed between the NB groups and the amino groups of the tissue (Bottom panel). The figure shows the proposed mechanism of action at the wound site. The hydrogel patch first seals the wound, then degrades with the infiltration of macrophages, leaving behind a pro-regenerative ambiance for tissue repair. (E) Confocal immunofluorescence images have been presented here. Red fluorescence corresponds to the expression of CD68 macrophages (M0 phenotype). The cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) represented in blue. Lastly, the expression of CD206 macrophages (M2 phenotype) has been shown with green fluorescence. The dashed line shows the boundary of the sample that was implanted in vitro. The increased expression of CD206 suggests tissue regeneration, whereas the downregulation of CD68 implies anti-inflammatory behavior. The corresponding graph quantifies the expression of CD206 with the designed patch and the commercially available glue, cyanoacrylate. There was a significant increase in this M2 macrophage marker up to 4 weeks post-treatment, indicating enhanced therapy with the designed patch. Reproduced with permission.167 Copyright 2022, Wiley-VCH GmbH.
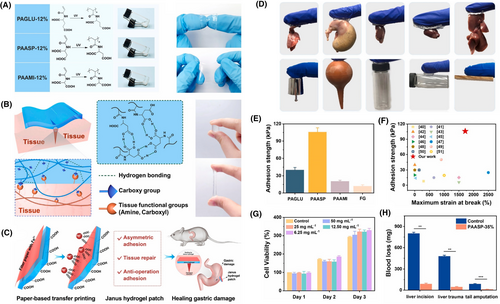
Adhesive hydrogel patch for repairing gastric perforation. (A) Schematic highlights the free radical polymerization involved in the synthesis of poly (N-acryloyl glutamic acid) (PAGLU), Poly (N-acryloyl aspartic acid) (PAASP), and poly (N-acryloyl amidomalonic acid) (PAAMI) hydrogels. Although all three bi-carboxyl-included vinyl polymers can form hydrogels, PAASP showed excellent adhesive behavior. It was hypothesized that the carboxyl groups of the aspartic acid could interact with the polar groups present at the tissue interfaces by hydrogen bonding, which leads to the material being adhesive. Corresponding images display all three fabricated hydrogels. (B) Figure illustrates the mechanism of PAASP adhesion to tissue surfaces. (C) Schematic shows the design of PAASP-based janus hydrogel patch for treating gastric perforation. (D) Photographs show the adhesion of the designed hydrogel patch to various tissue surfaces. (E) Graph compares the mechanical strength of the designed PAASP hydrogel patch. (F) Chart displays the superior adhesive strength of the designed patch compared to those found in the literature. (G) Graph demonstrates the viability of fibroblast cells when exposed to PAASP. (H) Plot shows the ability of PAASP in preventing in vivo blood loss. Reproduced with permission.168 Copyright 2022, Elsevier.
Another diverse area of application could involve the delivery of genetic material using sticky patches. In the post-genomic era, such nucleic acid-based therapies, especially non-coding ribonucleic acids (ncRNAs), are gradually gaining attention.169 These molecules are capable of modulating gene expression at both transcriptional and post-transcriptional levels. Among the different ncRNAs, microRNAs have found application in various biomedical applications, including chronic wound healing.170, 171 Micro RNAs are short (∼22 nucleotides) molecules that suppress the expression of target messenger RNAs via silencing or inducing transcript degradation, typically by binding to the 3′ UTR region of the target transcripts. Hydrogel patches can provide a suitable platform for delivering such genetic materials. For instance, Qu and coworkers designed a gelatin-based microneedle patch to deliver nucleic acids.172 However, the delivery of nucleic acids using hydrogel patches is in its infancy, and further research is needed to validate this technology.
Beyond diverse applications, advanced computational strategies could be implemented in the fabrication of hydrogel patches that demonstrate superior adhesion. High-throughput theoretical predictions, such as machine learning-based algorithms, can design and develop biomaterials for preparing hydrogel patches.173 Recently, Shi et al. developed a machine learning-based algorithm to predict the adhesive energy between sequence-specific polymers and patterned surfaces.174 In another instance, Rajabifar et al. designed an algorithm to predict the adhesive forces of polymers.175 Being able to predict the adhesive energy of polymers will allow for the rapid screening of sequences to obtain hydrogel patches with superior adhesive properties. Moreover, future studies should incorporate nanoparticles into these simulations to evaluate their effect on the adhesivity of the polymers. Such high-throughput algorithms will reduce the time, resources, and costs of preparing the scaffolds. Moreover, at molecular levels, these algorithms can manipulate the chemical and physical structure of the biomaterials to generate unique favorable physicochemical scaffold properties, such as viscoelastic behavior, mechanical strength, toughness, and response to pH changes, among others. For instance, Li et al. used linear and non-linear classification algorithms to screen through a library of peptides to predict and fabricate hydrogel peptides at neutral pH using metal ion-based crosslinkers.176 Specifically, they were able to quantitatively determine the relationship between the chemical structure and gelation behavior of the designed hydrogels. Zhang et al., on the other hand, used a physics-guided machine learning approach to determine the rheological properties of the designed hydrogels rapidly.177 Additionally, the sustained release of pharmaceutical therapeutic molecules can be predicted and programmed using machine learning.178, 179 Castro et al. used an artificial neural network to predict the release profiles of drug molecules from 3D printed hydrogel constructs.180 Such powerful computational tools will be used more often in the future to design advanced hydrogel patch-based drug delivery systems.
To conclude, in this review, we tried to identify and discuss the unique material properties and fabrication techniques that are currently playing critical roles in advancing hydrogel patches for various wound healing and tissue regenerative applications. In addition, we discussed the various material functionalities introduced by nanoparticles to these adhesive patches. Ultimately, understanding the fundamental mechanism of cell-material interactions, further development of advanced fabrication strategies, and using multifunctional nanoparticles enabled by advanced computational tools will lead to the discovery of the next generation of adhesive hydrogel patches suitable for diverse biomedical applications.
ACKNOWLEDGMENTS
A.P. is thankful for the funding and support from Canada Research Chairs Program of the Natural Sciences and Engineering Research Council (NSERC) of Canada, NSERC Discovery Accelerator Supplements (DAS), NSERC Discovery Grant, Early Research Award (ERA) from Province of Ontario, New Frontiers in Research Fund (NFRF)-Exploration Stream, The Center for Advanced Materials and Biomaterials Research (CAMBR) Seed Grant, Wolfe-Western Fellowship At-Large for Outstanding Newly Recruited Research Scholar, and CIHR (IMHA Priority Announcement) Project Grant. The authors would also like to acknowledge BioRender; some images and illustrations were created with BioRender.com.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Biographies

Dr. Aishik Chakraborty is a Postdoctoral Associate in the Department of Chemical and Biochemical Engineering at The University of Western Ontario, Canada. His expertise lies in various microfabrication strategies, including 3D bioprinting. Currently, he is developing novel polymeric biomaterial-based platforms for tissue engineering and drug delivery applications. He also specializes in nanotechnology, fabricating smart stimuli-responsive nanocomposite polymeric scaffolds for on-demand drug release.
Dr. Chakraborty received his M.S. and Ph.D. degrees from the Department of Chemical and Petroleum Engineering at The University of Kansas, USA. His graduate research involved evaluating the behavior of biomolecules at air/water interfaces.

Dr. Sudhir Ranganath is an Assistant Professor in the Department of Chemical Engineering and founder of the Laboratory for Biomedical Innovations via Engineering & NanoTechnology (Bio-INvENT Lab) at Siddaganga Institute of Technology, India. He was the INSA Visiting Scientist at IIT Bombay in 2023 and a Roche Collaborative Research Fellow at the Indiana University School of Optometry, USA in 2021. He received his B.E in Chemical Engineering from Bangalore University, M.S., & Ph.D., in Chemical Engineering from the National University of Singapore and postdoctoral experience at Harvard-MIT Division of Health Sciences & Technology, USA & JNCASR, India. He is a member of Sigma Xi (USA), ARVO (USA) and CRS (USA) and a life member of Institute of Engineers (India).

Dr. Subrata Chakrabarti obtained MBBS from BSMC. Following a DO in Calcutta University, he moved to Canada. He obtained M.S. and Ph.D. at the University of Manitoba and did residency training in anatomical pathology. Following a fellowship at Yale University, he joined University of Western Ontario, Canada. Currently, he is a distinguished University professor and an anatomical Pathologist at London Health Sciences Centre. Dr. Chakrabarti is an active educator and served as Chair/Chief of Pathology and Laboratory Medicine at Western University and London Health Sciences Centre from 2011-2021.
Dr. Chakrabarti’s research focuses on diabetic retinopathy and other chronic diabetic complications. His current research focuses on epigenetics and non-coding RNAs.

Dr. Arghya Paul is an Associate Professor and Canada Research Chair Tier II in Advanced Cell-Instructive Materials and Biotherapeutics at the University of Western Ontario. Professor Paul received his Ph.D. in Biomedical Engineering from McGill University in 2012 and postdoctoral training at Harvard-MIT Division of Health Sciences and Technology, prior to starting his independent research career in 2014. Paul’s Biointel Laboratory focuses on engineering new class of biomimetic nano delivery systems and hydrogels for controlled drug delivery and translational research in medicine.




