The posterior neural plate in axolotl can form mesoderm or neuroectoderm
Abstract
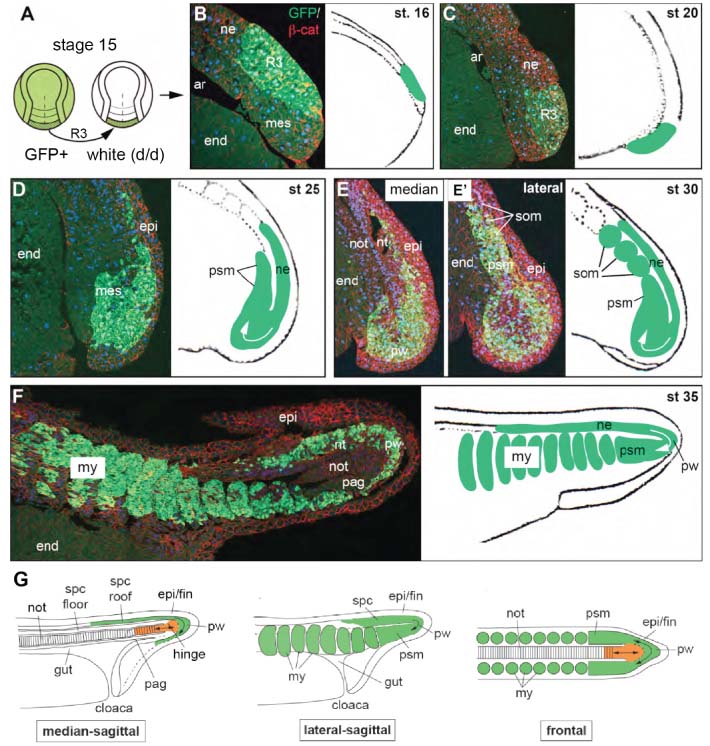
Gastrulation is the developmental process where germ layers are formed. It was generally assumed that germ layer specification is finished by the end of gastrulation. However, previous studies in amphibians revealed that posterior neural plate and fold, although traditionally regarded as neural, have a mesodermal bias and give rise to tail and posterior trunk muscles whereas only more anterior regions give rise to spinal chord [1-4]. In addition, recent studies in zebrafish, chick, and mouse revealed bipotential stem cell populations at the posterior ends of these embryos. The stem cells can give rise to neural and mesodermal tissues depending on local signaling in the tail bud [5-7]. Here, we reinvestigated morphogenesis and fate of the posterior neural plate in an amphibian model, the axolotl (Ambystoma mexicanum), using GFP-labeled grafts of the posterior third of the neural plate (reg. 3; stage 15; Figure 1) for detailed lineage analysis. In situ hybridisation with riboprobes against mesodermal (brachyury, bra) and neuronal/stem cell (sox2) markers revealed an ambiguous determinative state of reg. 3 at the time of transplantation. While its central and posterior part is bra-positive, small left and right anterior regions expressed sox2. The more anterior plate regions 2 and 1 are sox2-positive and bra-negative. The border of the two markers is ill defined and shows some overlap. Histological analysis of reg. 3 grafts at early tailbud stages revealed that the cells of the most posterior part of reg. 3 start moving from dorsal to ventral forming the posterior wall. Then they turn anteriorly, become connected with paraxial presomitic mesoderm and form somites on either side of the embryo (Figure 1). As a result of this movement, the posterior half of reg. 3 gives rise to posterior trunk and anterior tail somites whereas the anterior half forms posterior tail somites and tail spinal cord. Only cells that conduct this anterior turn and pass the Wnt/b-catenin positive posterior wall will contribute to paraxial mesoderm. Cells that remain in the dorsal part of the tail-bud eventually form lateral and dorsal parts of the tail spinal chord. The floor plate of the posterior spinal chord and the axial mesoderm do not contain any reg. 3 cells which indicates that they may be formed from the chordoneural hinge, a connection between the posteriormost reg. 2 cells and the tip of the notochord [8]. Additional grafting experiments showed that the notochord is formed from axial mesoderm that was already involuted during gastrulation and underwent massive elongation, presumably by convergence and extension. Therefore, axial and paraxial mesoderm of the tail are formed by different mechanisms and are probably specified at different time points, i.e. during gastrulation and tail bud development.
Taken together, these data show that germ layer specification is not complete after gastrulation, but that part of the putative neural plate is specified during morphogenetic movements of tail bud stages in order to become paraxial mesoderm.
Figure