Zorro: multi-reference dose-fractionated image registration
Abstract
The technique of single-particle analysis (SPA) in cryo-TEM has recently been revolutionized by the simultaneous introduction of direct electron detectors (DEDs) and maximum likelihood, reference-free reconstruction tools such as Relion [S. Scheres] and Bayesian refinement tools such as Frealign [N. Grigorieff]. CMOS DEDs have much better DQE than scintillator-coupled CCDs and fast read-out permits collection of dose-fractionated image stacks which can be effectively drift-corrected. SPA is essentially an ensemble-average in-line holography technique. Particles (i.e. proteins) are embedded in a vitreous ice matrix, such that they have random projections. Many micrographs are recorded at various defocus values (typically 1.0 – 2.5 µm). With sufficient particle projections recorded (typically 50k-500k), it is possible to estimate orientations of the particles by maximum likelihood iterative refinement and reconstruct the 3-D volume via back-projection using the central projection theorem.
Proteins are highly radiation sensitive (critical dose ~20 e−/Å2 = 0.03 C/cm2) therefore useful per-frame dose is in the range 0.5 – 2 e−/Å2. Ice films are viscous and thought to be semi-insulating under electron illumination. High, charged-driven drift rates are observed (x10 compared to hard specimens on carbon). Radiation damage and non-rigid particle motion results in rapid decay of correlation information amongst frames. In addition, DED's gain reference change quickly due to radiation damage, such that correlated noise is often greater than the correlated signal.
We will present Zorro, a new drift registration package, to be open-sourced (github.com/C-CINA/zorro) in the near-future after publication. Zorro takes a similar multiple reference approach as Motioncorr [Y. Cheng], in that it overcomes noise by cross-correlating each frame to ~10 frames of similar dose, resulting in an over-determined set of image shifts. In Zorro the error amongst image shifts is optimized by a global-minimizer, the Basin hopping algorithm. The individual correlations are logistic-weighted in the minimizer, based on the statistical significance of the correlation peak compared to the background noise. To deconvolve the impact of correlated noise shared between frames, a masked intensity-normalized cross-correlation (MNXC) is used. The MNXC algorithm also deconvolves non-uniform ice thickness and illumination.
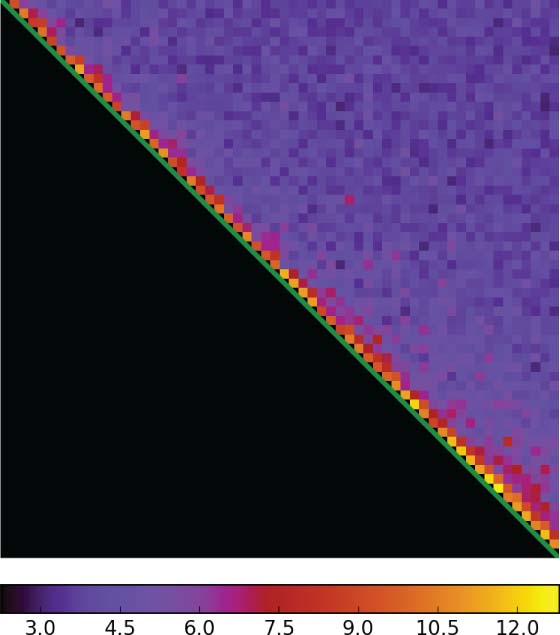
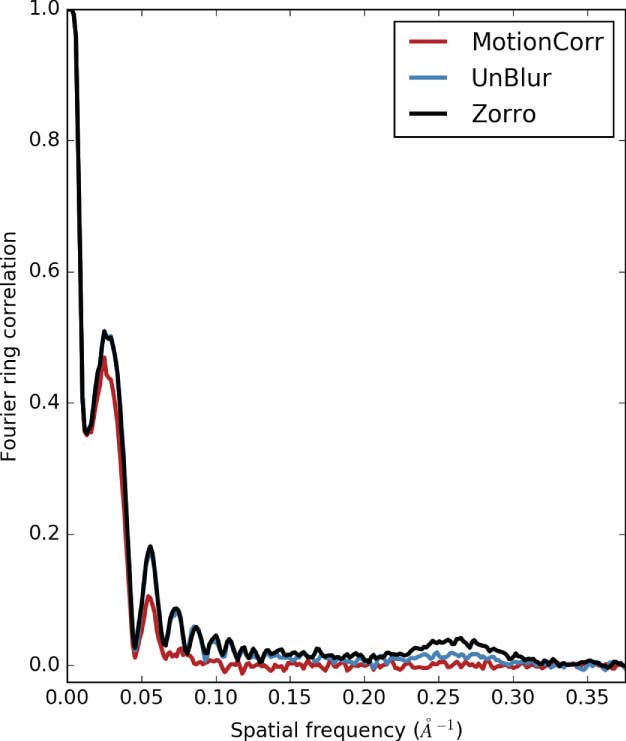
A test specimen of urease particles are shown in Fig. 1 with (top) single frames with dose 0.3 e−/Å2 and (bottom) the aligned sum of 60 frames. The set of correlations can be represented by an upper triangular matrix (Fig. 2), where the diagonal represents the frame number and the horizontal the adjacent cross-correlation maxima. The correlations rapidly drop to the noise level after the adjacency exceeds frames due to non-rigid particle motion and radiation damage. Registration success at low-resolution may be assessed by independently registering the even- and odd-numbered frames, and calculating the cross-correlation between the independent halves. The normalized, rotationally averaged correlation, known as the Fourier Ring Correlation (FRC), is shown in Fig.3 for a small (145 kDa) protein. FRC oscillations are due to defocus.
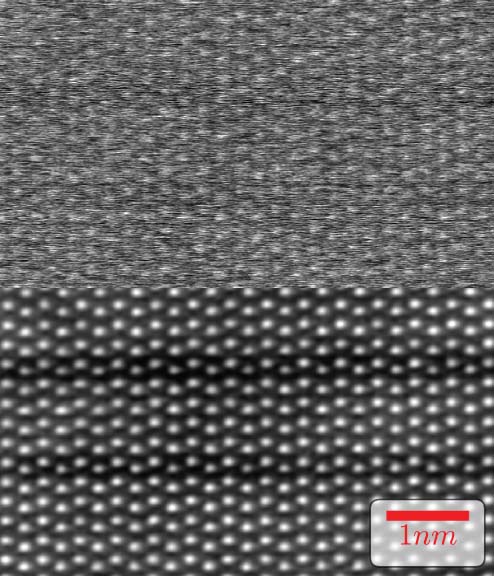
Zorro is a general-purpose algorithm without technique-specific heuristics. Here we show it applied to dose-fractionated HAADF-STEM. Shown in Fig. 4, a (top) rapid-scan rate with 100 ns dwell can be combined with dose-fractionation to (bottom) dampen scan errors in the sum.
Figure captions

Acknowledgements
Julia Kowal recorded the image used for the FRC in Fig. 3. Kenneth Goldie and Ariane Fecteau-Lefebvre are thanked for maintaining the TEM instruments.



