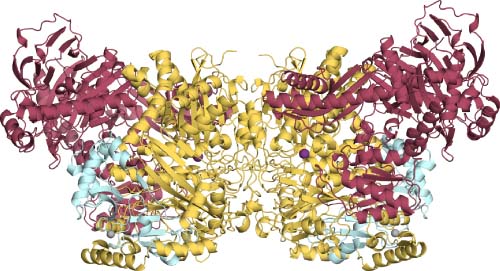
Acetone Carboxylase
Abstract
Carboxylases incorporate CO2, or its hydrated form HCO3−, into organic substrates. Assimilatory carboxylases combine CO2 to a diversity of carbon sources allowing for growth on intransigent, poorly activated organic compounds such as acetone. Acetone carboxylase catalyzes the adenosine triphosphate-dependent carboxylation of acetone to acetoacetate. Acetone carboxylase undergoes a large conformational shift to allow phosphorylated intermediates, carboxyphosphate and phosphenolacetone to interact with the Mn bound active site. The coordinated Mn orients the intermediates for the carboncarbon bond formation producing acetoacetate. Biochemical, molecular, structural, and spectroscopic studies have shown how acetone carboxylase functions and gives clues on how this unique biotin-independent carboxylation mechanism couples ATP hydrolysis to bicarbonate-dependent carboxylation. This article highlights the body of biochemical, spectroscopic, and structural work leading to insights into the catalytic mechanism of acetone carboxylase.
3D Structure