Highly Enantioselective Inverse-Electron-Demand Hetero-Diels–Alder Reactions of α,β-Unsaturated Aldehydes†
Karl Gademann Dr.
Department of Chemistry and Chemical Biology Harvard University Cambridge, MA 02138 (USA) Fax: (+1) 617-496-1880
Search for more papers by this authorDavid E. Chavez
Department of Chemistry and Chemical Biology Harvard University Cambridge, MA 02138 (USA) Fax: (+1) 617-496-1880
Search for more papers by this authorEric N. Jacobsen Prof. Dr.
Department of Chemistry and Chemical Biology Harvard University Cambridge, MA 02138 (USA) Fax: (+1) 617-496-1880
Search for more papers by this authorKarl Gademann Dr.
Department of Chemistry and Chemical Biology Harvard University Cambridge, MA 02138 (USA) Fax: (+1) 617-496-1880
Search for more papers by this authorDavid E. Chavez
Department of Chemistry and Chemical Biology Harvard University Cambridge, MA 02138 (USA) Fax: (+1) 617-496-1880
Search for more papers by this authorEric N. Jacobsen Prof. Dr.
Department of Chemistry and Chemical Biology Harvard University Cambridge, MA 02138 (USA) Fax: (+1) 617-496-1880
Search for more papers by this authorThis work was supported by the NIH (GM-59316), with additional support from the Schweizerischer Nationalfonds zur Förderung der wissenschaftlichen Forschung (postdoctoral fellowship to K.G.), and the National Science Foundation (predoctoral fellowship to D.E.C.). We are grateful to Dr. R. Staples for carrying out the X-ray crystal structure analysis of 1.
Graphical Abstract
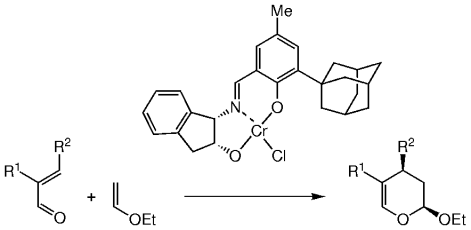
Straightforward access to useful synthetic intermediates is provided by this new method. Simple, α,β-unsaturated aldehydes are excellent substrates in the hetero-Diels–Alder reaction with inverse electron demand, catalyzed by CrIII–Schiff base complexes (see scheme; R1, R2=alkyl or aryl) in the presence of 4-Å molecular sieves and no solvent. The resulting dihydropyrans are obtained in high enantio- (89–98 % ee) and diastereoselectivity (>95 % de) and yield (40–95 %).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2002/z19516_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. F. Tietze, G. Kettschau, J. A. Gewart, A. Schuffenhauer, Curr. Org. Chem. 1998, 2, 19–62;
- 1bL. Tietze, G. Kettschau, Top. Curr. Chem. 1997, 189, 1–120;
- 1cD. L. Boger in Comprehensive Organic Synthesis, Vol. 5 (Ed ); Pergamon, Oxford, 1991, pp. 451–512;
- 1dD. L. Boger, S. M. Weinreb, Hetero Diels–Alder Methodology in Organic Synthesis, Academic Press, San Diego, 1987.
- 2See, for example
- 2aR. R. Schmidt, M. Maier, Tetrahedron Lett. 1985, 26, 2065–2068;
- 2bR. R. Schmidt, B. Haag-Zeino, M. Hoch, Liebigs Ann. Chem. 1988, 885–889;
- 2cG. Dujardin, S. Molato, E. Brown, Tetrahedron: Asymmetry 1993, 4, 193–196;
- 2dL. F. Tietze, A. Montenbruck, C. Schneider, Synlett 1994, 509–510;
- 2eL. F. Tietze, C. Schneider, C. A. Grote, Chem. Eur. J. 1996, 2, 139–148;
- 2fG. Dujardin, S. Rossignol, E. Brown, Tetrahedron 1996, 52, 4007–4010.
- 3
- 3aSulfone-activated oxabutadienes: E. Wada, H. Yasuoka, S. Kanemasa, Chem. Lett. 1994, 9, 1637–1640; E. Wada, W. Pei, H. Yasuoka, U. Chin, S. Kanemasa, Tetrahedron 1996, 52, 1205–1220;
- 3bphosphonate-activated oxabutadienes: D. A. Evans, J. S. Johnson, J. Am. Chem. Soc. 1998, 120, 4895–4896;
- 3ccarboxylic ester-activated oxabutadienes: J. Thorhauge, M. Johannsen, K. A. Jørgensen, Angew. Chem. 1998, 110, 2543–2546;
10.1002/(SICI)1521-3757(19980904)110:17<2543::AID-ANGE2543>3.0.CO;2-P Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2404–2406;10.1002/(SICI)1521-3773(19980918)37:17<2404::AID-ANIE2404>3.0.CO;2-D CAS PubMed Web of Science® Google ScholarD. A. Evans, E. J. Olhava, J. S. Johnson, J. M. Janey, Angew. Chem. 1998, 110, 3554–3557; Angew. Chem. Int. Ed. 1998, 37, 3372–3375;10.1002/(SICI)1521-3773(19981231)37:24<3372::AID-ANIE3372>3.0.CO;2-K CAS PubMed Web of Science® Google ScholarD. A. Evans, J. S. Johnson, E. J. Olhava, J. Am. Chem. Soc. 2000, 122, 1635–1649; H. Audrain, J. Thorhauge, R. G. Hazell, K. A. Jørgensen, J. Org. Chem. 2000, 65, 4487–4497.
- 4In particular, this pattern of substitution is found in iridoid and pheromone natural products. For examples, see: Comprehensive Natural Products Chemistry, Vol. 8 (Ed ), Pergamon-Elsevier, Oxford, 1999, pp. 197–373.
10.1016/B978-0-08-091283-7.00052-7 Google Scholar
- 5A. G. Dossetter, T. F. Jamison, E. N. Jacobsen, Angew. Chem. 1999, 111, 2549–2552;
10.1002/(SICI)1521-3757(19990816)111:16<2549::AID-ANGE2549>3.0.CO;2-H Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2398–2400.10.1002/(SICI)1521-3773(19990816)38:16<2398::AID-ANIE2398>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 6For applications of closely related catalysts to asymmetric hetero-ene reactions, see: R. T. Ruck, E. N. Jacobsen, J. Am. Chem. Soc. 2002, 124, 2882–2883.
- 7R. I. Longley, W. S. Emerson, J. Am. Chem. Soc. 1950, 72, 3079–3081; C. W. Smith, D. G. Norton, S. A. Ballard, J. Am. Chem. Soc. 1951, 73, 5267–5270; B. B. Snider, G. B. Phillips, J. Org. Chem. 1983, 48, 2789–2792. Danishefsky et al. and Spino et al. have noted the acceleration of this reaction by achiral Lewis acids: S. J. Danishefsky, M. Bednarski, Tetrahedron Lett. 1984, 25, 721–724; C. Spino, L. Clouston, D. J. Berg, Can. J. Chem. 1996, 74, 1762–1764; C. Spino, L. L. Clouston, D. J. Berg, Can. J. Chem. 1997, 75, 1047–1054.
- 8A variety of chiral tridentate (Schiff base)chromium complexes were evaluated, and complex 1 afforded the best results with regard both to enantioselectivity and reactivity.
- 9No exo diastereoisomer could be detected by analysis of the crude reaction mixture by 1H NMR spectroscopy.
- 10The use of excess ethyl vinyl ether had a beneficial effect on conversion and prevented aldehyde self-condensation in the case of substrates with aliphatic β substituents.
- 11D. P. Steinhuebel, J. J. Fleming, J. Du Bois, Org. Lett. 2002, 4, 293–295.
- 12J. B. Jones, A. J. Irwin, J. Am. Chem. Soc. 1977, 99, 556; D. A. Evans, J. S. Johnson, E. J. Olhava, J. Am. Chem. Soc. 2000, 122, 1635–1649.
- 13This class of compounds can be prepared by desymmetrization of the cyclic anhydrides, for examples see: Y. Cheng, L. Deng, J. Am. Chem. Soc. 2000, 122, 9542–9543; R. Verma, S. Mithram, S. K. Ghosh, J. Chem. Soc. Perkin Trans. 1 1999, 257–264; G. Jaeschke, D. Seebach, J. Org. Chem. 1998, 63, 1190–1193; P. D. Theisen, C. H. Heathcock, J. Org. Chem. 1993, 58, 142–156; K. Yamamoto, T. Nishioka, J. Oda, Y. Yamamoto, Tetrahedron Lett. 1988, 29, 1717–1720.
- 14Full experimental details are provided as Supporting Information.
- 15Improvement in catalyst performance extends to normal-demand HDA reactions as well. For example, the reaction of 1-benzyloxybutadiene with 3-(triisopropylsilyl)propynal produced cycloadduct in 89 % ee using catalyst prepared by the original procedure, and 94 % ee using the new catalyst. This reaction served as the starting point for the synthesis of fostriecin: D. E. Chavez, E. N. Jacobsen, Angew. Chem. 2001, 113, 3779–3782;
10.1002/1521-3757(20011001)113:19<3779::AID-ANGE3779>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3667–3670.10.1002/1521-3773(20011001)40:19<3667::AID-ANIE3667>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 16Details of the crystal structure analysis are provided as Supporting Information. CCDC-189470 (1) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 17R. T. Ruck, E. N. Jacobsen, unpublished results.
- 18No cycloaddition is observed in the absence of dessicant. For a discussion on a related system, see ref [6].



41:16<>1.0.co;2-7.cover.gif)
