Observation of the Direct Products of Migratory Insertion in Aryl Palladium Carbene Complexes and Their Subsequent Hydrolysis†
Ana C. Albéniz Dr.
Departamento de Química Inorgánica Facultad de Ciencias, Universidad de Valladolid Prado de la Magdalena s/n, 47005 Valladolid (Spain) Fax: (+34) 983-42-013
Search for more papers by this authorPablo Espinet Prof.
Departamento de Química Inorgánica Facultad de Ciencias, Universidad de Valladolid Prado de la Magdalena s/n, 47005 Valladolid (Spain) Fax: (+34) 983-42-013
Search for more papers by this authorRaúl Manrique
Departamento de Química Inorgánica Facultad de Ciencias, Universidad de Valladolid Prado de la Magdalena s/n, 47005 Valladolid (Spain) Fax: (+34) 983-42-013
Search for more papers by this authorAlberto Pérez-Mateo
Departamento de Química Inorgánica Facultad de Ciencias, Universidad de Valladolid Prado de la Magdalena s/n, 47005 Valladolid (Spain) Fax: (+34) 983-42-013
Search for more papers by this authorAna C. Albéniz Dr.
Departamento de Química Inorgánica Facultad de Ciencias, Universidad de Valladolid Prado de la Magdalena s/n, 47005 Valladolid (Spain) Fax: (+34) 983-42-013
Search for more papers by this authorPablo Espinet Prof.
Departamento de Química Inorgánica Facultad de Ciencias, Universidad de Valladolid Prado de la Magdalena s/n, 47005 Valladolid (Spain) Fax: (+34) 983-42-013
Search for more papers by this authorRaúl Manrique
Departamento de Química Inorgánica Facultad de Ciencias, Universidad de Valladolid Prado de la Magdalena s/n, 47005 Valladolid (Spain) Fax: (+34) 983-42-013
Search for more papers by this authorAlberto Pérez-Mateo
Departamento de Química Inorgánica Facultad de Ciencias, Universidad de Valladolid Prado de la Magdalena s/n, 47005 Valladolid (Spain) Fax: (+34) 983-42-013
Search for more papers by this authorThis work was supported by the Dirección General de Enseñanza Superior (Ministerio de Educación y Cultura, BQU2001-2015) and the Junta de Castilla y León (VA80/99 and VA17/00B). A fellowship from the Junta de Castilla y León to A.P.-M. is also acknowledged.
Graphical Abstract
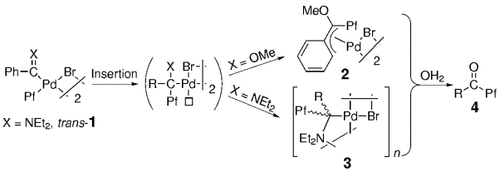
Die Wechselwirkung eines elektrophilen Carben-Kohlenstoffatoms mit der π-Elektronendichte einer Arylgruppe führt dazu, dass 1 bevorzugt im Sinne einer intramolekularen Insertion reagiert (siehe Schema; Pf=C6F5). Die mit dieser Reaktion zugänglichen Alkylverbindungen (2 oder 3) können identifiziert werden, bevor sie letztendlich zu 4 hydrolysieren.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author. Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2002/z18657_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Recent examples: Heck-type and coupling reactions:
- 1a W. A. Herrmann, V. P. W. Böhm, C. W. K. Gstöttmayr, M. Grosche, C.-P. Reisinger, T. Weskamp, J. Organomet. Chem. 2001, 617–618, 616–628, and references therein;
- 1b A. M. Magill, D. S. McGuinness, K. J. Cavell, G. J. P. Britovsek, V. C. Gibson, A. J. P. White, D. J. Williams, A. H. White, B. W. Skelton, J. Organomet. Chem. 2001, 617–618, 546–560;
- 1c E. Peris, J. A. Loch, J. Mata, R. H. Crabtree, Chem. Commun. 2001, 201–202;
- 1d J. Huang, S. P. Nolan, J. Am. Chem. Soc. 1999, 121, 9889–9890; amination reactions:
- 1e S. R. Stauffer, S. Lee, J. P. Stambuli, S. I. Hauck, J. F. Hartwig, Org. Lett. 2000, 2, 1423–1426;
- 1f J. Huang, G. Grasa, S. P. Nolan, Org. Lett. 1999, 1, 1307–1309; asymmetric hydrosilation:
- 1g D. Enders, H. Gielen, J. Organomet. Chem. 2001, 617–618, 70–80; dehalogenation:
- 1h M. S. Viciu, G. A. Grasa, S. P. Nolan, Organometallics 2001, 20, 3607–3612; copolymerization:
- 1i M. G. Gardiner, W. A. Herrmann, C.-P. Reisinger, J. Schwarz, M. Spiegel, J. Organomet. Chem. 1999, 572, 239–247.
- 2 For example, dimerization of a carbene ligand in Group 6 metal–carbene complexes is catalyzed by a diverse range of Pd catalysts: M. A. Sierra, J. C. Amo, M. J. Mancheño, M. Gómez-Gallego, J. Am. Chem. Soc. 2001, 123, 851–861.
- 3 D. S. McGuinness, N. Saendig, B. F. Yates, K. J. Cavell, J. Am. Chem. Soc. 2001, 123, 4029–4040.
- 4 D. Bourissou, O. Guerret, F. P. Gabbaï, G. Bertrand, Chem. Rev. 2000, 100, 39–91.
- 5
- 5a J. Cámpora, S. A. Hudson, P. Massiot, C. M. Maya, P. Palma, E. Carmona, L. A. Martínez-Cruz, A. Vegas, Organometallics 1999, 18, 5225–5237;
- 5b B. Crociani, R. L. Richards, J. Organomet. Chem. 1978, 154, 65–78, and references therein.
- 6 Some examples: cyclopropanation:
- 6a F. Bernardi, A. Bottoni, G. P. Miscione, Organometallics 2001, 20, 2751–2758;
- 6b C. Rodríguez-García, A. Oliva, R. M. Ortuño, V. Branchadell, J. Am. Chem. Soc. 2001, 123, 6157–6163, and references therein; cine substitution in Stille couplings:
- 6c C. A. Busacca, J. Swestock, R. E. Johnson, T. R. Bailey, L. Musza, C. A. Rodger, J. Org. Chem. 1994, 59, 7553–7556; other processes:
- 6d S. Ogoshi, T. Nishida, T. Shinagawa, H. Kurosawa, J. Am. Chem. Soc. 2001, 123, 7164–7165;
- 6e
M. Yoshida, M. Ihara, Angew. Chem. 2001, 113, 636–639;
Angew. Chem. Int. Ed. 2001, 40, 616–619;
10.1002/1521-3773(20010202)40:3<616::AID-ANIE616>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 6f B. M. Trost, G. J. Tanoury, J. Am. Chem. Soc. 1988, 110, 1636–1638;
- 6g B. M. Trost, C. R. Self, J. Am. Chem. Soc. 1983, 105, 5942–5944.
- 7 S.-T. Liu, K. R. Reddy, Chem. Soc. Rev. 1999, 28, 315–322.
- 8 Several geometrical isomers are possible for complexes 3. In CDCl3, 3 is a mixture of trans-anti and trans-syn isomers, which correspond to a trans arrangement of carbene moieties along the Pd⋅⋅⋅Pd axis and a relative syn or anti arrangement of the diethylamino groups relative to the coordination plane. The compound first formed is tentatively assigned (the assignment might be reversed) as trans-3, which (both isomers) slowly isomerizes to the cis (syn and anti isomers) and eventually undergoes hydrolysis when kept in solution for several days.
- 9 Decomposition data for 3 (%) after seven days in CDCl3 at room temperature: trans-3 (25.9), cis-3 (26.9), 5 (19.5), 7 (27.7).
- 10
- 10a A. C. Albéniz, P. Espinet, Y.-S. Lin, Organometallics 1996, 15, 5003–5009;
- 10b R. Usón, J. Forniés, P. Espinet, E. Lalinde, A. García, P. G. Jones, K. Meyer-Bäse, G. M. Sheldrick, J. Chem. Soc. Dalton Trans. 1986, 259–264.
- 11
- 11a A. C. Albéniz, P. Espinet, Y.-S. Lin, Organometallics 1997, 16, 4030–4032;
- 11b L. E. Crascall, J. L. Spencer, J. Chem. Soc. Dalton Trans. 1992, 3445–3452.
- 12 The 19F NMR spectrum of 6 shows two broad signals for the two inequivalent Fortho atoms at δ=−135.93 and −127.54 ppm, which indicate there is restricted rotation of the Pf group about the CC bond, and hence the Pf group must be located in a sterically crowded position. The chemical shifts for these signals are consistent with a Pf group bound to a carbon atom and closely influenced by the metal center, which also points to an anti arrangement of the group.
- 13 8: R. Usón, J. Forniés, P. Espinet, R. Navarro, E. Lalinde, Trans. Met. Chem. 1984, 9, 277–279. Both 8 and 9 are a mixture of atropisomers in solution at room temperature which are derived from hindered rotation about both C(carbene)N bonds.
- 14 E. O. Fischer, F. R. Kreibl, Synthetic Methods of Organometallic and Inorganic Chemistry, Thieme, Stuttgart, 1997, pp. 129–131.
- 15 E. O. Fischer, U. Schubert, H. Fischer, Inorg. Synth. 1979, 19, 169–171.
- 16 A. C. Albéniz, P. Espinet, C. Foces-Foces, F. H. Cano, Organometallics 1990, 9, 1079–1085.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:13<>1.0.co;2-j.cover.gif)
