Comparison of Reorganization Energies for Intra- and Intermolecular Electron Transfer†
Hiroshi Imahori Prof. Dr.
Department of Molecular Engineering Graduate School of Engineering Kyoto University PRESTO, JAPAN Science and Technology Corporation Sakyo-ku, Kyoto 606-8501 (Japan) Fax: (+81) 75-751-7279
Search for more papers by this authorHiroko Yamada Dr.
Department of Material and Life Science Graduate School of Engineering Osaka University CREST, JAPAN Science and Technology Corporation Suita, Osaka 565-0871 (Japan) Fax: (+81) 6-6879-7370
Search for more papers by this authorDirk M. Guldi Dr. habil.
Radiation Laboratory University of Notre Dame Notre Dame, Indiana 46556 (USA) Fax: (+)574-631-8068
Search for more papers by this authorYoshito Endo
Department of Material and Life Science Graduate School of Engineering Osaka University CREST, JAPAN Science and Technology Corporation Suita, Osaka 565-0871 (Japan) Fax: (+81) 6-6879-7370
Search for more papers by this authorAkihisa Shimomura
Department of Chemistry Graduate School of Engineering Science and Research Center for Materials Science at Extreme Conditions Osaka University Toyonaka, Osaka 560-8531 (Japan) Fax: (+81)6-6850-6244
Search for more papers by this authorSanti Kundu Dr.
Department of Chemistry Graduate School of Engineering Science and Research Center for Materials Science at Extreme Conditions Osaka University Toyonaka, Osaka 560-8531 (Japan) Fax: (+81)6-6850-6244
Search for more papers by this authorKoji Yamada Dr.
The Institute of Scientific and Industrial Research Osaka University Mihoga-oka, Ibaraki, Osaka 567-0047 (Japan)
Search for more papers by this authorTadashi Okada Prof. Dr.
Department of Chemistry Graduate School of Engineering Science and Research Center for Materials Science at Extreme Conditions Osaka University Toyonaka, Osaka 560-8531 (Japan) Fax: (+81)6-6850-6244
Search for more papers by this authorYoshiteru Sakata Prof. Dr.
The Institute of Scientific and Industrial Research Osaka University Mihoga-oka, Ibaraki, Osaka 567-0047 (Japan)
Search for more papers by this authorShunichi Fukuzumi Prof. Dr.
Department of Material and Life Science Graduate School of Engineering Osaka University CREST, JAPAN Science and Technology Corporation Suita, Osaka 565-0871 (Japan) Fax: (+81) 6-6879-7370
Search for more papers by this authorHiroshi Imahori Prof. Dr.
Department of Molecular Engineering Graduate School of Engineering Kyoto University PRESTO, JAPAN Science and Technology Corporation Sakyo-ku, Kyoto 606-8501 (Japan) Fax: (+81) 75-751-7279
Search for more papers by this authorHiroko Yamada Dr.
Department of Material and Life Science Graduate School of Engineering Osaka University CREST, JAPAN Science and Technology Corporation Suita, Osaka 565-0871 (Japan) Fax: (+81) 6-6879-7370
Search for more papers by this authorDirk M. Guldi Dr. habil.
Radiation Laboratory University of Notre Dame Notre Dame, Indiana 46556 (USA) Fax: (+)574-631-8068
Search for more papers by this authorYoshito Endo
Department of Material and Life Science Graduate School of Engineering Osaka University CREST, JAPAN Science and Technology Corporation Suita, Osaka 565-0871 (Japan) Fax: (+81) 6-6879-7370
Search for more papers by this authorAkihisa Shimomura
Department of Chemistry Graduate School of Engineering Science and Research Center for Materials Science at Extreme Conditions Osaka University Toyonaka, Osaka 560-8531 (Japan) Fax: (+81)6-6850-6244
Search for more papers by this authorSanti Kundu Dr.
Department of Chemistry Graduate School of Engineering Science and Research Center for Materials Science at Extreme Conditions Osaka University Toyonaka, Osaka 560-8531 (Japan) Fax: (+81)6-6850-6244
Search for more papers by this authorKoji Yamada Dr.
The Institute of Scientific and Industrial Research Osaka University Mihoga-oka, Ibaraki, Osaka 567-0047 (Japan)
Search for more papers by this authorTadashi Okada Prof. Dr.
Department of Chemistry Graduate School of Engineering Science and Research Center for Materials Science at Extreme Conditions Osaka University Toyonaka, Osaka 560-8531 (Japan) Fax: (+81)6-6850-6244
Search for more papers by this authorYoshiteru Sakata Prof. Dr.
The Institute of Scientific and Industrial Research Osaka University Mihoga-oka, Ibaraki, Osaka 567-0047 (Japan)
Search for more papers by this authorShunichi Fukuzumi Prof. Dr.
Department of Material and Life Science Graduate School of Engineering Osaka University CREST, JAPAN Science and Technology Corporation Suita, Osaka 565-0871 (Japan) Fax: (+81) 6-6879-7370
Search for more papers by this authorThis work was supported by COE, Grant-in-Aid for Scientific Research, Specially Promoted Research (No. 10102007), and the Development of Innovative Technology (No. 12310) from Ministry of Education, Sports, Culture, Science and Technology, Japan and by the Office of Basic Energy Sciences of the U.S. Department of Energy. This is document NDRL-4379 from the Notre Dame Radiation Laboratory.
Graphical Abstract
Bevorzugen Elektronen Ebenen oder Kugeln? Die Reorganisationsenergien für den intramolekularen Elektronentransfer unter Beteiligung eines drei- (C60) oder zweidimensionalen Acceptors (NIm; siehe Schema) wurden bestimmt. Ein Vergleich der Reorganisationsenergien für den intra- und intermolekularen Elektronentransfer lieferte erstmals einen Einblick in den Zusammenhang zwischen intrinsischen Reorganisationsenergien und unterschiedlichen Molekülgeometrien.
Supporting Information
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author. Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2001/2002/z18508_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a R. A. Marcus, N, Sutin, Biochim. Biophys. Acta 1985, 811, 265;
- 1b R. A. Marcus, Angew. Chem. 1993, 105, 1161; Angew. Chem. Int. Ed. Engl. 1993, 32, 1111;
- 1c J. R. Winkler, H. B. Gray, Chem. Rev. 1992, 92, 369;
- 1d H. B. Gray, J. R. Winkler in Electron Transfer in Chemistry, Vol. 3 ( ), Wiley-VCH, Weinheim, 2001, pp. 3–23;
- 1e M. D. Newton, Chem. Rev. 1991, 91, 767;
- 1f M. D. Newton, Adv. Chem. Phys. 1999, 106, 303.
- 2 The Photosynthetic Reaction Center ( ), Academic Press, San Diego, 1993.
- 3
- 3a H. Imahori, N. V. Tkachenko, V. Vehmanen, K. Tamaki, H. Lemmetyinen, Y. Sakata, S. Fukuzumi, J. Phys. Chem. A 2001, 105, 1750;
- 3b V. Vehmanen, N. V. Tkachenko, H. Imahori, S. Fukuzumi, H. Lemmetyinen, Spectrochim. Acta Part A 2001, 57, 2229;
- 3c H. Imahori, K. Tamaki, D. M. Guldi, C. Luo, M. Fujitsuka, O. Ito, Y. Sakata, S. Fukuzumi, J. Am. Chem. Soc. 2001, 123, 2607.
- 4 Reorganization energies of diporphyrin dyads for intramolecular ET have been reported. However, no quantitative comparison of the λ values with the corresponding 2D acceptors has been performed.
- 4a A. Harriman, V. Heitz, J.-P. Sauvage, J. Phys. Chem. 1993, 97, 5940;
- 4b J.-C. Chambron, A. Harriman, V. Heitz, J.-P. Sauvage, J. Am. Chem. Soc. 1993, 115, 7419;
- 4c J. M. DeGraziano, P. A. Liddell, L. Leggett, A. L. Moore, T. A. Moore, D. Gust, J. Phys. Chem. 1994, 98, 1758;
- 4d A. Osuka, G. Noya, S. Taniguchi, T. Okada, Y. Nishimura, I. Yamazaki, N. Mataga, Chem. Eur. J. 2001, 7, 3134.
- 5a H. Imahori, K. Hagiwara, T. Akiyama, M. Aoki, S. Taniguchi, T. Okada, M. Shirakawa, Y. Sakata, Chem. Phys. Lett. 1996, 263, 545;
- 5b D. Kuciauskas, P. A. Liddell, S. Lin, S. G. Stone, A. L. Moore, T. A. Moore, D. Gust, J. Phys. Chem. B 2000, 104, 4307;
- 5c
A. Nakano, A. Osuka, T. Yamazaki, Y. Nishimura, S. Akimoto, I. Yamazaki, A. Itaya, M. Murakami, H. Miyasaka, Chem. Eur. J. 2001, 7, 3134.
10.1002/1521-3765(20010716)7:14<3134::AID-CHEM3134>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 6 Farid and Harriman indicated that the rate of charge recombination was affected by the size of the aromatic nucleus. It is highly likely that the effect is a result of changes in reorganization energy. I. R. Gould, D. Ege, J. E. Moser, S. Farid, J. Am. Chem. Soc. 1990, 112, 4290; A. M. Brun, A. Harriman, S. M. Stephan, J. Phys. Chem. 1992, 96, 254.
- 7 Although free rotations around the spacer methylene unit in ZnP-NIm and the pyrolidine moiety in ZnP-C60 are possible, such rotations would not affect the ET rates as much as reported in similar porphyrin–quinone systems. T. Asahi, M. Ohkochi, R. Matsusaka, N. Mataga, R. P. Zhang, A. Osuka, K. Maruyama, J. Am. Chem. Soc. 1993, 115, 5665.
- 8 H. Imahori, K. Tamaki, H. Yamada, K. Yamada, Y. Sakata, Y. Nishimura, I. Yamazaki, M. Fujitsuka, O. Ito, Carbon 2000, 38, 1599.
- 9 K. Yamada, H. Imahori, Y. Nishimura, I. Yamazaki, Y. Sakata, Chem. Lett. 1999, 895.
- 10 C. Luo, D. M. Guldi, H. Imahori, K. Tamaki, Y. Sakata, J. Am. Chem. Soc. 2000, 122, 6535.
- 11
- 11a G. P. Wiederrecht, M. P. Niemczyk, W. A. Svec, M. R. Wasielewski, J. Am. Chem. Soc. 1996, 118, 81;
- 11b R. T. Hayes, M. R. Wasielewski, D. Gosztola, J. Am. Chem. Soc. 2000, 122, 5563.
- 12 The Marcus plots for ZnP-CONH-C60 involving CS from the excited porphyrin and C60 singlet states, and CS from the excited C60 triplet state, and CR from C60.− to ZnP.+ have been reported.[3c] The plots in THF, PhCN, and DMF fit well to a single parabolic Marcus curve on the basis of Equation (1), which implies that the classical Marcus theory is valid for both the CS and CR processes in the porphyrin–fullerene linked systems. This result supports the idea that reorganization energies of porphyrin–fullerene systems are virtually the same in the three solvents because of the charge delocalization in porphyrin and fullerene moieties, which would mask the solvent effects on the reorganization energies.[3c,4d] Thus, the λ values are assumed to be similar in ZnP-NHCO-C60, ZnP-CC-C60, and ZnP-CONH-C60.
- 13 The V values of ZnP-NHCO-C60, ZnP-CC-C60, ZnP-CONH-C60 (7.9±1.7 cm−1), and ZnP-NIm (7.8±3.2 cm−1) are found to be similar. This result agrees with the similarity in the intervening spacer moieties. We have also reported the linear correlation of logarithmic kET versus edge-to-edge distance (Ree) in porphyrin–fullerene linked systems (i.e., porphyrin–fullerene dyads, triads, and tetrad) including ZnP-CONH-C60 where the decay coefficient factor (β) is 0.6 Å−1. Since the Ree values (11.9 Å) are the same in the two systems, the similar V values are consistent with the distance dependence of kET. H. Imahori, D. M. Guldi, K. Tamaki, Y. Yoshida, Y. Sakata, S. Fukuzumi, J. Am. Chem. Soc. 2001, 123, 6617.
- 14 S. Fukuzumi, I. Nakanishi, T. Suenobu, K. M. Kadish, J. Am. Chem. Soc. 1999, 121, 3468.
- 15 S. Marguet, P. Hapiot, P. Neta, J. Phys. Chem. 1994, 98, 7136.
- 16 R. Chang, J. Chem. Educ. 1970, 47, 563.
- 17 The same hyperfine coupling constants give a good fit for the ESR spectra in the presence of NIm-ref using different ΔBpp values. Thus, the observed line broadening because of electron self-exchange was in the slow-exchange region.[16]
- 18
The kex value was determined using equation: kex=1.52×108 (ΔBpp−ΔB
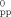 )/{(1−Pi)[NIm-ref]}, where ΔBpp and ΔB
)/{(1−Pi)[NIm-ref]}, where ΔBpp and ΔB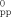 are the peak-to-peak line widths of the ESR spectra in the presence and absence of NIm-ref, respectively, and Pi is a statistical factor which can be taken as nearly zero.[16]
are the peak-to-peak line widths of the ESR spectra in the presence and absence of NIm-ref, respectively, and Pi is a statistical factor which can be taken as nearly zero.[16]
- 19 H. Imahori, K. Tamaki, Y. Araki, T. Hasobe, O. Ito, A. Shimomura, S. Kundu, T. Okada, Y. Sakata, S. Fukuzumi, J. Phys. Chem. A 2002, 106, 2803.
- 20 S. Fukuzumi, I. Nakanishi, K. Tanaka, T. Suenobu, A. Tabard, R. Guilard, E. V. Caemelbecke, K. M. Kadish, J. Am. Chem. Soc. 1999, 121, 785.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:13<>1.0.co;2-j.cover.gif)
