Enantioselective Synthesis of Kedarcidin Chromophore Aglycon in Differentially Protected Form
Andrew G. Myers Prof.
Department of Chemistry and Chemical Biology Harvard University Cambridge, Massachusetts 02138 (USA) Fax: (+1) 617-495-4976
Search for more papers by this authorPhilip C. Hogan
Department of Chemistry and Chemical Biology Harvard University Cambridge, Massachusetts 02138 (USA) Fax: (+1) 617-495-4976
Search for more papers by this authorAlexander R. Hurd
Department of Chemistry and Chemical Biology Harvard University Cambridge, Massachusetts 02138 (USA) Fax: (+1) 617-495-4976
Search for more papers by this authorSteven D. Goldberg
Department of Chemistry and Chemical Biology Harvard University Cambridge, Massachusetts 02138 (USA) Fax: (+1) 617-495-4976
Search for more papers by this authorAndrew G. Myers Prof.
Department of Chemistry and Chemical Biology Harvard University Cambridge, Massachusetts 02138 (USA) Fax: (+1) 617-495-4976
Search for more papers by this authorPhilip C. Hogan
Department of Chemistry and Chemical Biology Harvard University Cambridge, Massachusetts 02138 (USA) Fax: (+1) 617-495-4976
Search for more papers by this authorAlexander R. Hurd
Department of Chemistry and Chemical Biology Harvard University Cambridge, Massachusetts 02138 (USA) Fax: (+1) 617-495-4976
Search for more papers by this authorSteven D. Goldberg
Department of Chemistry and Chemical Biology Harvard University Cambridge, Massachusetts 02138 (USA) Fax: (+1) 617-495-4976
Search for more papers by this authorFinancial support from the National Institutes of Health is gratefully acknowledged. We thank Dr. Richard Staples and Mr. Andrew Haidle for the X-ray analysis of compound 20.
Graphical Abstract
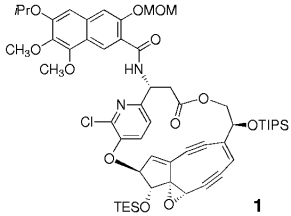
Vier in Grammmengen hergestellte Komponenten wurden verknüpft bei der konvergenten (längste lineare Sequenz: 25 Stufen), enantioselektiven Synthese des komplementär geschützten Aglycons des Kedarcidin-Chromophors (1). Einer der Schlüsselschritte war eine transanulare anionisch induzierte Cyclisierung zum Bicyclo[7.3.0]dodecadiendiin-Gerüst in Gegenwart eines überbrückten Makrolactons. MOM=Methoxymethyl, TIPS=Triisopropylsilyl, TES=Triethylsilyl.
References
- 1 Isolation:
- 1a K. S. Lam, G. A. Hesler, D. R. Gustavson, A. R. Crosswell, J. M. Veitch, S. Forenza, K. Tomita, Antibiotics 1991, 44, 472;
- 1b S. J. Hofstead, J. A. Matson, A. R. Malacko, H. Marquardt, Antibiotics 1992, 45, 1250; characterization and structure proposal:
- 1c J. E. Leet, D. R. Schroeder, S. J. Hofstead, J. Golik, K. L. Colson, S. Huang, S. E. Klohr, T. W. Doyle, J. A. Matson, J. Am. Chem. Soc. 1992, 114, 7946;
- 1d J. E. Leet, D. R. Schroeder, D. R. Langley, K. L. Colson, S. Huang, S. E. Klohr, M. S. Lee, J. Golik, S. J. Hofstead, T. W. Doyle, J. A. Matson, J. Am. Chem. Soc. 1993, 115, 8432; structure revision:
- 1e S. Kawata, S. Ashizawa, M. Hirama, J. Am. Chem. Soc. 1997, 119, 12 012.
- 2 Neocarzinostatin: The Past, Present, and Future of an Anticancer Drug ( ), Springer, Tokyo, 1997.
- 3 K.-i. Yoshida, Y. Minami, R. Azuma, M. Saeki, T. Otani, Tetrahedron Lett. 1993, 34, 2637.
- 4 H. Chimura, M. Ishizuka, M. Hamada, S. Hori, K. Kimura, J. Inawaga, T. Takeuchi, H. Umezawa, J. Antibiot. 1968, 21, 44.
- 5 A. S. Khokhlov, B. Z. Cherches, P. D. Reshetov, G. M. Smirnova, I. B. Sorokina, T. A. Prokoptzeva, T. A. Koloditskaya, V. V. Smirnov, J. Antibiot. 1969, 22, 541.
- 6 D. R. Schroeder, K. L. Colson, S. E. Klohr, N. Zein, D. R. Langley, M. S. Lee, J. A. Matson, T. W. Doyle, J. Am. Chem. Soc. 1994, 116, 9351.
- 7 Review: K. C. Nicolaou, W.-M. Dai, Angew. Chem. 1991, 103, 1453; Angew. Chem. Int. Ed. Engl. 1991, 30, 1387.
- 8a S. Kawata, F. Yoshimura, J. Irie, H. Ehara, M. Hirama, Synlett 1997, 250;
- 8b F. Yoshimura, S. Kawata, M. Hirama, Tetrahedron Lett. 1999, 40, 8281;
- 8c
M. J. Lear, F. Yoshimura, M. Hirama, Angew. Chem. 2001, 113, 972;
10.1002/1521-3757(20010302)113:5<972::AID-ANGE972>3.0.CO;2-0 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 946.10.1002/1521-3773(20010302)40:5<946::AID-ANIE946>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 9
A. G. Myers, S. D. Goldberg, Angew. Chem. 2000, 112, 2844;
10.1002/1521-3757(20000804)112:15<2844::AID-ANGE2844>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2732.10.1002/1521-3773(20000804)39:15<2732::AID-ANIE2732>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 10 Reactive (Z)-enediyne synthesis by dehydration:
- 10a R. R. Jones, R. G. Bergman, J. Am. Chem. Soc. 1972, 94, 660;
- 10b T. Doi, T. Takahashi, J. Org. Chem. 1991, 56, 3465;
- 10c K. Iida, M. Hirama, J. Am. Chem. Soc. 1995, 117, 8875; see also: ref. [9].
- 11
- 11a
C. Glaser, Ber. Dtsch. Chem. Ges. 1869, 2, 422; for a recent review, see:
10.1002/cber.186900201183 Google Scholar
- 11b
P. Siemsen, R. C. Livingston, F. Diederich, Angew. Chem. 2000, 112, 2740;
10.1002/1521-3757(20000804)112:15<2740::AID-ANGE2740>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2632.10.1002/1521-3773(20000804)39:15<2632::AID-ANIE2632>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 12 A. G. Myers, Y. Horiguchi, Tetrahedron Lett. 1997, 38, 4363.
- 13
- 13a R. A. Singer, E. M. Carreira, W. Lee, J. Am. Chem. Soc 1994, 116, 8837; review:
- 13b E. M. Carreira, R. A. Singer, Drug Discovery Today 1996, 1, 145.
- 14 D. G. Wishka, D. R. Graber, E. P. Seest, L. A. Dolak, F. Han, W. Watt, J. Morris, J. Org. Chem. 1998, 63, 7851.
- 15 For reviews, see:
- 15a O. Mitsunobu, Synthesis 1981, 1;
- 15b D. L. Hughes, Org. React. 1992, 42, 335.
- 16 For a convenient in situ protocol, see: E. Fabiano, B. T. Golding, M. M. Sadeghi, Synthesis 1987, 190.
- 17 K. Iida, M. Hirama, J. Am. Chem. Soc. 1994, 116, 10 310.
- 18 B. M. Kim, K. B. Sharpless, Tetrahedron Lett. 1989, 30, 655.
- 19 E. J. Corey, A. Venkateswarlu, J. Am. Chem. Soc. 1972, 94, 6190.
- 20 Examples of stereoselective Pd-mediated coupling reactions of 1,1-dibromoolefins, Z-selective:
- 20a J. M. Nuss, R. A. Rennels, B. H. Levine, J. Am. Chem. Soc. 1993, 115, 6991;
- 20b S. Torii, H. Okumoto, T. Tadokoro, A. Nishimura, M. A. Rashid, Tetrahedron Lett. 1993, 34, 2139; E-selective:
- 20c W. R. Roush, K. J. Moriarty, B. B. Brown, Tetrahedron Lett. 1990, 31, 6509;
- 20d W. Shen, L. Wang, J. Org. Chem. 1999, 64, 8873;
- 20e J. Uenishi, K. Matsui, Tetrahedron Lett. 2001, 42, 4353; see also: ref. [9]; for E-selective couplings of 1,1-dichloroolefins with organozinc and organomagnesium reagents, see:
- 20f A. Minato, K. Suzuki, K. Tamao, J. Am. Chem. Soc. 1987, 109, 1257.
- 21 J. Inanaga, K. Hirata, H. Saeki, T. Katsuki, M. Yamaguchi, Bull. Chem. Soc. Jpn. 1979, 52, 1989. Our conditions differ from those previously reported in that the macrolactonization is carried out by slow addition of a solution of the hydroxy acid to a suspension of trichlorobenzoyl chloride, triethylamine, and DMAP in toluene at 50 °C.
- 22 K. Jones, M. F. Lappert, J. Chem. Soc. 1965, 1944.
- 23
- 23a J. K. Stille, Angew. Chem. 1986, 98, 504; Angew. Chem. Int. Ed. Engl. 1986, 25, 508;
- 23b J. K. Stille, J. H. Simpson, J. Am. Chem. Soc. 1987, 109, 2138;
- 23c V. Farina, Pure Appl. Chem. 1996, 68, 73.
- 24 This compound was observed to be a mixture of two equilibrating conformational isomers (atropisomers) in solution. A detailed analysis of the thermodynamics and kinetics of this process is presented elsewhere.[25]
- 25 A. G. Myers, A. R. Hurd, P. C. Hogan, J. Am. Chem. Soc., submitted.
- 26 G. Eglinton, A. R. Galbraith, J. Chem. Soc. 1959, 889.
- 27 Compounds 2, 3, 4, and 22 were not stable in neat form, but could be stored as dilute solutions in toluene at −20 °C. Yields were obtained using an internal standard (1H NMR).
- 28 This compound exists as a single atropisomeric structure in solution. A detailed analysis of the thermodynamics and kinetics of this process is presented elsewhere.[25]
- 29 J. C. Martin, R. J. Arhart, J. Am. Chem. Soc. 1971, 93, 4327.
- 30
- 30a W. Zhang, J. L. Loebach, S. R. Wilson, E. N. Jacbosen, J. Am. Chem. Soc. 1990, 112, 2801; review:
- 30b E. N. Jacobsen in Catalytic Asymmetric Synthesis ( ), VCH, New York, 1993, chap. 4.2.
- 31 Y. Hoshino, H. Yamamoto, J. Am. Chem. Soc. 2000, 122, 10 452.
- 32 S. Kanemoto, T. Nonaka, K. Oshima, K. Utimoto, H. Nozaki, Tetrahedron Lett. 1986, 27, 3387.
- 33 Prepared by the method of D. E. Bissing, C. A. Matuszak, W. E. McEwen, J. Am. Chem. Soc. 1964, 86, 3824.
- 34 Prepared by the method of W. H. Richardson, V. F. Hodge, J. Org. Chem. 1970, 35, 4012.
- 35 Use of cumene hydroperoxide led to a mixture of 22 and 24 in which the undesired epoxide 22 is favored.
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



114:6<>1.0.co;2-7.cover.gif)
