High-Valent Manganese Corroles and the First Perhalogenated Metallocorrole Catalyst
Galina Golubkov
Department of Chemistry and Institute of Catalysis Science and Technology Technion—Israel Institute of Technology Haifa 32000 (Israel) Fax: (+972) 4-8233735
Search for more papers by this authorJesper Bendix Dr.
Beckman Institute California Institute of Technology Pasadena, CA 91125 (USA) Fax: (+1) 626 449 4159
Search for more papers by this authorHarry B. Gray Prof. Dr.
Beckman Institute California Institute of Technology Pasadena, CA 91125 (USA) Fax: (+1) 626 449 4159
Search for more papers by this authorAtif Mahammed Dr.
Department of Chemistry and Institute of Catalysis Science and Technology Technion—Israel Institute of Technology Haifa 32000 (Israel) Fax: (+972) 4-8233735
Search for more papers by this authorIsrael Goldberg Prof. Dr.
School of Chemistry Tel Aviv University Tel Aviv 69978 (Israel) Fax: (+972) 3-6409293
Search for more papers by this authorAngel J. DiBilio Dr.
Beckman Institute California Institute of Technology Pasadena, CA 91125 (USA) Fax: (+1) 626 449 4159
Search for more papers by this authorZeev Gross Prof. Dr.
Department of Chemistry and Institute of Catalysis Science and Technology Technion—Israel Institute of Technology Haifa 32000 (Israel) Fax: (+972) 4-8233735
Search for more papers by this authorGalina Golubkov
Department of Chemistry and Institute of Catalysis Science and Technology Technion—Israel Institute of Technology Haifa 32000 (Israel) Fax: (+972) 4-8233735
Search for more papers by this authorJesper Bendix Dr.
Beckman Institute California Institute of Technology Pasadena, CA 91125 (USA) Fax: (+1) 626 449 4159
Search for more papers by this authorHarry B. Gray Prof. Dr.
Beckman Institute California Institute of Technology Pasadena, CA 91125 (USA) Fax: (+1) 626 449 4159
Search for more papers by this authorAtif Mahammed Dr.
Department of Chemistry and Institute of Catalysis Science and Technology Technion—Israel Institute of Technology Haifa 32000 (Israel) Fax: (+972) 4-8233735
Search for more papers by this authorIsrael Goldberg Prof. Dr.
School of Chemistry Tel Aviv University Tel Aviv 69978 (Israel) Fax: (+972) 3-6409293
Search for more papers by this authorAngel J. DiBilio Dr.
Beckman Institute California Institute of Technology Pasadena, CA 91125 (USA) Fax: (+1) 626 449 4159
Search for more papers by this authorZeev Gross Prof. Dr.
Department of Chemistry and Institute of Catalysis Science and Technology Technion—Israel Institute of Technology Haifa 32000 (Israel) Fax: (+972) 4-8233735
Search for more papers by this authorThis research (No. 368/00) was supported by the Israel Science Foundation (Z.G.), the US National Science Foundation (H.B.G.), and the Fund for the Promotion of Research at the Technion (Z.G.).
Graphical Abstract
Eine Alternative zu Porphyrinsystemen bieten Corrol-Liganden. Die Mangancorrole 1–4 beispielsweise sind leicht zugänglich, Redoxreaktionen finden bei ihnen am Metallzentrum (nicht am Liganden) statt, und insbesondere 4 zeigt beeindruckende katalytische Aktivität bei der Epoxidierung von Styrol mit Iodosylbenzol.
References
- 1
- 1a
B. Meunier in Metalloporphyrins Catalyzed Oxidations ( ), Kluwer, Dordrecht, 1994, pp. 1–48;
10.1007/978-94-017-2247-6_1 Google Scholar
- 1b J. T. Groves in Cytochrome P450: Structure, Mechanism, and Biochemistry ( ), 2nd ed., Plenum, New York, 1995, chap. 1.
- 2
- 2a D. Mansuy, Coord. Chem. Rev. 1993, 125, 129;
- 2b Z. Gross, L. Simkhovich, Tetrahedron Lett. 1998, 39, 8171.
- 3 J. T. Groves, T. Takahashi, J. Am. Chem. Soc. 1983, 105, 2073.
- 4 N. Jin, J. T. Groves, J. Am. Chem. Soc. 1999, 121, 2923.
- 5 H. Volz, W. Muller, Chem. Ber. 1997, 130, 1099, and references therein.
- 6
- 6a Y. Watanabe, H. Fujii, Struct. Bonding 2000, 97, 61;
- 6b L. Kaustov, M. E. Tal, A. I. Shames, Z. Gross, Inorg. Chem. 1997, 36, 3503.
- 7
- 7a
S. Will, J. Lex, E. Vogel, H. Schmickler, J. P. Gisselbrecht, C. Haubtmann, M. Bernard, M. Gross, Angew. Chem. 1997, 109, 367;
10.1002/ange.19971090410 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 357, and refs therein;
- 7b Tetrahedron Organic Chemistry Series, Vol. 15: Expanded, Contracted & Isomeric Porphyrins ( ), Pergamon, UK, 1997, chap. 1.
- 8
- 8a S. Licoccia, E. Morgante, R. Paolesse, F. Polizio, M. O. Senge, E. Tondello, T. Boschi, Inorg. Chem. 1997, 36, 1564;
- 8b R. Paolesse, L. Jaquinod, M. O. Senge, K. M. Smith, J. Org. Chem. 1997, 62, 6193;
- 8c K. M. Kadish, V. A. Adamian, E. Van Caemelbecke, E. Gueletii, S. Will, C. Erben, E. Vogel, J. Am. Chem. Soc. 1998, 120, 11 986.
- 9 For data on manganese(IV) complexes of 2,3,7,8,12,13,17,18-octaethylcorrole (H3(oec)) see: C. Erben, S. Will, K. M. Kadish in The Porphyrin Handbook, Vol. 2 ( ), Academic Press, New York, 2000, chap. 12.
- 10
- 10a
Z. Gross, N. Galili, I. Saltsman, Angew. Chem. 1999, 111, 1530;
10.1002/(SICI)1521-3757(19990517)111:10<1530::AID-ANGE1530>3.0.CO;2-D Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 1427;10.1002/(SICI)1521-3773(19990517)38:10<1427::AID-ANIE1427>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 10b Z. Gross, N. Galili, L. Simkhovich, I. Saltsman, M. Botoshansky, D. Bläser, R. Boese, I. Goldberg, Org. Lett. 1999, 1, 599.
- 11
- 11a Z. Gross, L. Simkhovich, N. Galili, Chem. Commun. 1999, 599.
- 11b
Z. Gross, G. Golubkov, L. Simkhovich, Angew. Chem. Int. Ed. 2000, 39, 4045;
10.1002/1521-3773(20001117)39:22<4045::AID-ANIE4045>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 11c
L. Simkhovich, A. Mahammed, I. Goldberg, Z. Gross, Chem. Eur. J. 2001, 7, 1041.
10.1002/1521-3765(20010302)7:5<1041::AID-CHEM1041>3.0.CO;2-8 CAS PubMed Web of Science® Google Scholar
- 12
- 12a L. Simkhovich, N. Galili, I. Saltsman, I. Goldberg, Z. Gross, Inorg. Chem. 2000, 39, 2704;
- 12b A. E. Meier-Callahan, H. B. Gray, Z. Gross, Inorg. Chem. 2000, 39, 3604;
- 12c J. Bendix, H. B. Gray, G. Golubkov, Z. Gross, Chem. Commun. 2000, 1957.
- 13 S. Cai, F. A. Walker, S. Licoccia, Inorg. Chem. 2000, 39, 3466
- 14 2: A hexane solution of 1 (10 mg, 11.8 μmol) was treated with a hexane solution of Br2 (5.9 μmol), which resulted in quantitative precipitation of 2 as a red-brown solid. MS−: e/z (%): 927 (40) [M−], 848 (100) [M−−Br], UV/Vis (CH2Cl2) λmax [nm] (lg ε) 368 (4.63), 416 (4.72), 582 (3.74); elemental analysis (%) calcd for C37H8BrF15MnN4⋅2 H2O: C 46.08, H 1.25, N 5.81; found: C 45.76, H 1.38, N 6.20. Recrystallization from benzene/heptane resulted in X-ray quality crystals. [Mn(tpfc)(Br)], crystallized as benzene hemi-solvate (C37H8BrF15MnN4)⋅1/2 (C6H6): formula weight 967.38, monoclinic, space group P21/c, a=13.425(1), b=23.629(1), c=11.208(1) Å, β=98.38(1)°, V=3517.5(2) Å3, Z=4, T=110 K, ρcalcd=1.827 g cm−3, μ(MoKα)=1.63 mm−1, 6636 unique reflections, R1=0.053 for 4854 observations with Fo>4σ(Fo), R1=0.082 (wR2=0.148) for all unique data, |Δρ|=1.31 e Å−3.
- 15
3: A hexane solution of 1 (2.5 mg, 3 μmol) was treated with a dichloromethane solution of tris(4-bromophenyl)aminium hexachloroantimonate (2.5 mg, 3 μmol), which resulted in quantitative precipitation of 3 as a red-brown solid. MS−: e/z (%): 883 (25) [M−], 848 (100) [M−−Cl]; UV/Vis (CH2Cl2) λmax [nm] 362, 414 (Soret), 582 (Q band). Recrystallization from benzene/heptane resulted in X-ray quality crystals. [Mn(tpfc)(Cl)], crystallized as dibenzene solvate (C37H8ClF15MnN4)⋅2 (C6H6): formula weight 1040.08, triclinic, space group P
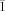 , a=8.547(1), b=13.488(1), c=19.675(1) Å, α=71.08(1), β=85.59(1), γ=73.88(1)°, V=2061.1(2) Å3, Z=2, T=110 K, ρcalcd=1.676 g cm−3, μ(MoKα)=0.50 mm−1, 7056 unique reflections, R1=0.062 for 4274 observations with Fo>4σ(Fo), R1=0.128 (wR2=0.147) for all unique data, |Δρ|=0.55 e Å−3. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-147467 (2) and CCDC-147468 (3). Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
, a=8.547(1), b=13.488(1), c=19.675(1) Å, α=71.08(1), β=85.59(1), γ=73.88(1)°, V=2061.1(2) Å3, Z=2, T=110 K, ρcalcd=1.676 g cm−3, μ(MoKα)=0.50 mm−1, 7056 unique reflections, R1=0.062 for 4274 observations with Fo>4σ(Fo), R1=0.128 (wR2=0.147) for all unique data, |Δρ|=0.55 e Å−3. Crystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-147467 (2) and CCDC-147468 (3). Copies of the data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: (+44) 1223-336-033; e-mail: [email protected]).
- 16 M. J. Camenzind, F. J. Hollander, C. L. Hill, Inorg. Chem. 1983, 22, 3776.
- 17 A. Tulinsky, B. M. L. Chen, J. Am. Chem. Soc. 1977, 99, 3647.
- 18 P. Turner, M. J. Gunter, B. W. Skelton, A. H. White, Aust. J. Chem. 1998, 51, 835.
- 19 J. DuBois, C. S. Tomooka, J. Hong, E. M. Carreira, Acc. Chem. Res. 1997, 30, 364.
- 20 [Mn(Br8tpfc)] 5: A solution of bromine (5 mL, 0.2 g, 1.2 mmol) and 1 (10 mg, 12 μmol) in methanol was stirred overnight, after which the methanol and excess bromine were removed under vacuum. Recrystallization from ethanol/water afforded 14.8 mg (85 % yield) of 5. MS−: e/z (%): 1479 (100) [M−], 1399 (10) [M−−Br]; UV/Vis (CH2Cl2) λmax [nm] 402 (4.73), 422 (4.70), 490 (4.38), 612 (4.24); 19F NMR (CDCl3): δ=−138 (two overlapping br s, 6 F), −152.3 (br s, 1 F), −153.2 (br s, 2 F), −160.5 (br s, 2 F), −161.4 (br s, 4 F).
Citing Literature
This is the
German version
of Angewandte Chemie.
Note for articles published since 1962:
Do not cite this version alone.
Take me to the International Edition version with citable page numbers, DOI, and citation export.
We apologize for the inconvenience.



113:11<>1.0.co;2-#.cover.gif)
