n-Butyllithium†
Abstract
[109-72-8] C4H9Li (MW 64.05)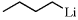
InChI = 1S/C4H9.Li/c1-3-4-2;/h1,3-4H2,2H3;
InChIKey = MZRVEZGGRBJDDB-UHFFFAOYSA-N
InChI = 1/C4H9.Li/c1-3-4-2;/h1,3-4H2,2H3;/rC4H9Li/c1-2-3-4-5/h2-4H2,1H3
InChIKey = MZRVEZGGRBJDDB-NESCHKHYAE
(strong base capable of lithiating carbon acids;1 useful for heteroatom-facilitated lithiations;2, 3 useful for lithium–halogen exchange;1, 4 reagent of choice for lithium–metal transmetalation reactions1a,c,5)
Physical Data: colorless liquid; stable at rt; eliminates LiH on heating; d25 0.765; mp −76 °C; bp 80–90 °C/0.0001 mmHg; dipole moment 0.97 D.6 13C NMR, 1H NMR, 6Li NMR8 and MS studies have been reported.7-9
Solubility: sol hydrocarbon and ethereal solvents, but should be used at low temperature in the latter solvent type: half-lives in diethyl ether and THF have been reported;10 reacts violently with H2O and other protic solvents.
Form Supplied in: commercially available as approximately 1.6 M, 2.5 M, and 10.0 M solution in hexanes and in cyclohexane, approximately 2.0 M solution in pentane, and approximately 1.7 M, and 2.7 M solution in n-heptane. Hexameric in hydrocarbons;1c tetrameric in diethyl ether;1c dimer–tetramer equilibrium mixture in THF;11 when used in combination with tertiary polyamines such as TMEDA and DABCO, reactivity is usually increased.1, 12
Analysis of Reagent Purity: since the concentration of commercial solutions may vary appreciably it is necessary to standardize solutions of the reagent prior to use. A recommended method for routine analyses involves titration of the reagent with s-butyl alcohol using 1,10-phenanthroline or 2,2′-biquinoline as indicator.15 Several other methods have been described.16
Preparative Methods: may be prepared in high yield from n-butyl chloride13 or n-butyl bromide14 and Lithium metal in ether or hydrocarbon solvents.
Handling, Storage, and Precautions: solutions of the reagent are pyrophoric and the reagent may catch fire if exposed to air or moisture. Handling of the reagent should be done behind a shield in a chemical fume hood. Safety goggles, chemical resistant gloves, and other protective clothing should be worn. In case of fire, a dry-powder extinguisher should be used: in no case should an extinguisher containing water or halogenated hydrocarbons be used to fight an alkyllithium fire. Bottles and reaction flasks containing the reagent should be flushed with N2 or preferably Ar and kept tightly sealed to preclude contact with oxygen or moisture. Standard syringe/cannula techniques for air and moisture sensitive chemicals should be applied when transferring the reagent. For detailed handling techniques see Wakefield.1b
Bibliography
- 1 (a) Wakefield, B. J. The Chemistry of Organolithium Compounds; Pergamon: Oxford, 1974. (b) Wakefield, B. J. Organolithium Methods; Academic: San Diego, 1990. (c) Wardell, J. L. In Comprehensive Organometallic Chemistry; G. Wilkinson, Ed.; Pergamon: Oxford, 1982; Chapter 2.
- 2 Gschwend, H. W.; Rodriguez, H. R., Org. React. 1979, 26, 1.
- 3 Krief, A., Tetrahedron 1980, 36, 2531.
- 4
Wardell, J. L. In
Inorganic Reactions and Methods;
J. J. Zuckerman, Ed.;
VCH: New York,
1988;
Vol. 11,
p
107.
10.1002/9780470145258.ch9 Google Scholar
- 5
Wardell, J. L. In
Inorganic Reactions and Methods;
J. J. Zuckerman, Ed.;
VCH:
New York,
1988;
Vol. 11,
p
31.
10.1002/9780470145258.ch7 Google Scholar
- 6 Dictionary of Organometallic Compounds; J. Buckingham, Ed.; Chapman & Hall: London, 1984; Vol. 1, p 1213.
- 7 (a) Seebach, D.; Hässig, R.; Gabriel, J., Helv. Chim. Acta 1983, 66, 308. (b) Heinzer, J.; Oth, J. F. M.; Seebach, D., Helv. Chim. Acta 1985, 68, 1848.
- 8 McGarrity, J. F., J. Am. Chem. Soc. 1985, 107, 1805, 1810.
- 9 Plavsik, D.; Srzic, D.; Klasinc, L., J. Phys. Chem. 1986, 90, 2075.
- 10 Bates, R. B.; Kroposki, L. M.; Potter, P. E., J. Org. Chem. 1972, 37, 560.
- 11 Bauer, W.; Clark, T.; Schleyer, P. v. R., J. Am. Chem. Soc. 1987, 109, 970.
- 12 (a) Langer, A. W., Adv. Chem. Ser. 1974, 130, 1. (b) Smith, W. N., Adv. Chem. Ser. 1974, 130, 23. (c) West, R., Adv. Chem. Ser. 1974, 130, 211.
- 13 (a) Bryce-Smith, D.; Turner, E. E., J. Chem. Soc 1953, 861. (b) Amonoo-Neizer, E. H.; Shaw, R. A.; Skovlin, D. O.; Smith, B. C., Inorg. Synth. 1966, 8, 19.
- 14 Jones, R. G.; Gilman, H., Org. React. 1951, 6, 339.
- 15 Watson, S. C.; Eastham, J. F., J. Organomet. Chem. 1967, 9, 165.
- 16 See e.g. (a) Gilman, H.; Haubein, A. H., J. Am. Chem. Soc. 1944, 66, 1515. (b) Gilman, H.; Cartledge, F. K., J. Organomet. Chem. 1964, 2, 447. (c) Eppley, R. L.; Dixon, J. A., J. Organomet. Chem. 1967, 8, 176. (d) Collins, P. F.; Kamienski, C. W.; Esmay, D. L.; Ellestad, R. B., Appl Catal 1961, 33, 468. (e) Lipton, M. F.; Sorensen, C. M.; Sadler, A. C.; Shapiro, R. H., J. Organomet. Chem. 1980, 186, 155. (f) Bergbreiter, D. E.; Pendergrass, E., J. Org. Chem. 1981, 46, 219.
- 17 Arnett, E. M.; Moe, K. D., J. Am. Chem. Soc. 1991, 113, 7068.
- 18 Meth-Cohn, O.; Gronowitz, S., J. Chem. Soc., Chem. Commun. 1966, 81.
- 19 Eisch, J. J., Org. Synth. 1981, 2, 98.
- 20 Herbrandson, H. F.; Mooney, D. S., J. Am. Chem. Soc. 1957, 79, 5809.
- 21
(a)
Takashi, K.;
Konishi, K.;
Ushio, M.;
Takaki, M.;
Asami, R.,
J. Organomet. Chem.
1973,
50,
1.
10.1016/S0022-328X(00)95081-2 Google Scholar(b) Kaiser, E. M.; McLure, J. R., J. Organomet. Chem. 1979, 175, 11.
- 22 Michelot, D.; Clinet, J.-C.; Linstrumelle, G., Synth. Commun. 1982, 12, 739.
- 23 (a) Bailey, W. F.; Ovaska, T. V., J. Am. Chem. Soc. 1993, 115, 3080. (b) Quillinan, A. J.; Scheinmann, F., org. Synth. 1978, 58, 1.
- 24 Rausch, M. D.; Ciappenelli, D. J., J. Organomet. Chem. 1967, 10, 127.
- 25 Klein, J.; Medlik-Balan, A., J. Am. Chem. Soc. 1977, 99, 1473.
- 26 Akiyama, S.; Hooz, J., Tetrahedron Lett. 1973, 4115.
- 27 Cardillo, G.; Contento, M.; Sandri, S., Tetrahedron Lett. 1974, 2215.
- 28 Schlosser, M.; Strunk, S., Tetrahedron Lett. 1984, 25, 741.
- 29 Faigl, F.; Schlosser, M., Tetrahedron Lett. 1991, 32, 3369.
- 30 (a) Heus-Kloos, Y. A.; de Jong, R. L. P.; Verkruisse, H. D.; Brandsma, L.; Julia, S., Synthesis 1985, 958. (b) Mordini, A.; Palio, G.; Ricci, A.; Taddei, M., Tetrahedron Lett. 1988, 29, 4991.
- 31 (a) Ahlbrecht, H.; Dollinger, H., Tetrahedron Lett. 1984, 25, 1353. (b) Hommes, H.; Verkruijsse, H. D.; Brandsma, L., Tetrahedron Lett. 1981, 22, 2495.
- 32 (a) Ahlbrecht, H., Chimia 1977, 31, 391. (b) Seebach, D.; Geiss, K.-H. In New Applications of Organometallic Reagents in Organic Synthesis; D. Seyferth, Ed.; Elsevier: Amsterdam, 1976; p 1.
- 33 Beak, P.; Zajdel, W. J.; Reitz, D. B., C.R. Hebd. Seances Acad. Sci. 1984, 84, 471.
- 34 Magnus, P., Tetrahedron 1977, 33, 2019.
- 35 Corey, E. J.; Seebach, D., J. Org. Chem. 1966, 31, 4097.
- 36 Durst, T. In Comprehensive Organic Chemistry; D. H. R. Barton; W. D. Ollis, Eds.; Pergamon: Oxford, 1979; Vol. 3, p 171.
- 37 Schlosser, M.; Schaub, B.; Spahic, B.; Sleiter, G., Helv. Chim. Acta 1973, 56, 2166.
- 38 Schoufs, M.; Meyer, J.; Vermeer, P.; Brandsma, L, Recl. Trav. Chim. Pays-Bas 1977, 96, 259.
- 39 Schlosser, M.; Ladenberger, V., Chem. Ber. 1967, 100, 3893.
- 40 Drakesmith, F. G.; Richardson, R. D. Stewart, O. J.; Tarrant, P., J. Org. Chem. 1968, 33, 286.
- 41
Schöllkopf, U.;
Stafforst, D.;
Jentsch, R.,
Liebigs Ann. Chem.
1977,
1167.
10.1002/jlac.197719770713 Google Scholar
- 42 Lehr, F.; Gonnermann, J.; Seebach, D., Helv. Chim. Acta 1979, 62, 2258.
- 43
(a)
Patterson, D. J.,
J. Organomet. Chem.
1967,
8,
199.
10.1016/S0022-328X(00)91032-5 Google Scholar(b) Appel, R.; Wander, M.; Knoll, F., Chem. Ber. 1979, 112, 1093. (c) Issleib, K.; Abicht, H. P., Prakt Chem 1970, 312, 456.
- 44 Maercker, A., Angew. Chem., Int. Ed. Engl. 1987, 26, 972.
- 45 Maercker, A.; Demuth, W., Liebigs Ann. Chem. 1977, 1909.
- 46 Bates, R. B.; Kroposki, L. M.; Potter, D. E., J. Org. Chem. 1972, 37, 560.
- 47 Gröbel, B. T.; Seebach, D., Synthesis 1977, 357.
- 48 Seebach, D.; Beck, A. K., Org. Synth., Coll. Vol. 1988, 6, 316.
- 49 Cere, V.; Paolucci, C.; Pollicino, S.; Sandri, E.; Fava, A., J. Org. Chem. 1991, 56, 4513.
- 50 Kodama, M.; Matsuki, Y.; Ito, S., Tetrahedron Lett. 1975, 3065.
- 51 Ramanathan, V.; Levine, R., J. Org. Chem. 1962, 27, 1216.
- 52 Labaudiniere, R.; Hilboll, G.; Leon-Lomeli, A.; Lautenschläger, H.-H.; Parnham, M.; Kuhl, P.; Dereu, N., J. Med. Chem. 1992, 35, 3156.
- 53
Schroeder, R.;
Schöllkopf, U.;
Blume, E.;
Hoppe, I.,
Liebigs Ann. Chem.
1975,
533.
10.1002/jlac.197519750315 Google Scholar
- 54 Chadwick, D. J.; Cliffe, I. A.; J. Chem. Soc., Perkin Trans. 1 1979, 2845.
- 55 Butler, D. E.; Alexander, S. M., J. Org. Chem. 1972, 37, 215.
- 56 Noyce, D. S.; Stowe, G. T., J. Org. Chem. 1973, 38, 3762.
- 57 Behringer, H. Ramert, R., Liebigs Ann. Chem. 1975, 1264.
- 58 Raap, R., Synlett 1971, 49, 2139.
- 59 Biellmann, J. F.; Ducep, J.-B., Org. React. 1982, 27, 1.
- 60
Yamamoto, Y.,
Comprehensive Organic Synthesis
1991,
1,
55.
10.1016/B978-0-08-052349-1.00024-X Google Scholar
- 61 Evans, D. A.; Andrews, G. C.; Buckwalter, B., J. Am. Chem. Soc. 1974, 96, 5560.
- 62 Stotter, P. L.; Hornish, R. E., J. Am. Chem. Soc. 1973, 95, 4444.
- 63 Hoppe, D.; Hanko, R.; Brönneke, A.; Lichtenberg, F.; Hülsen, E., Chem. Ber. 1985, 118, 2822.
- 64 Hoppe, D., Angew. Chem., Int. Ed. Engl. 1984, 23, 932.
- 65 Hoppe, D.; Hanko, R.; Brönneke, A.; Lichtenberg, F., Angew. Chem., Int. Ed. Engl. 1981, 20, 1024.
- 66 Beak, P.; Zajdel, W. J., J. Am. Chem. Soc. 1984, 106, 1010.
- 67 (a) Tischler, A. N.; Tischler, M. H., Tetrahedron Lett. 1978, 3. (b) Tischler, A. N.; Tischler, M. H., Tetrahedron Lett. 1978, 3407.
- 68 Savignac, P.; Leroux, Y.; Normant, H., Tetrahedron 1975, 31, 877.
- 69 Meyers, A. I.; Edwards, P. D.; Rieker, W. F.; Bailey, T. R., J. Am. Chem. Soc. 1984, 106, 3270.
- 70 (a) Gonzalez, M. A.; Meyers, A. I., Tetrahedron Lett. 1989, 30, 47. (b) Gonzalez, M. A.; Meyers, A. I., Tetrahedron Lett. 1989, 30, 43.
- 71 Gais, H. J.; Hellmann, G., J. Am. Chem. Soc. 1992, 114, 4439.
- 72 Hoppe, D.; Krämer, T., Angew. Chem., Int. Ed. Engl. 1986, 25, 160.
- 73 Rein, K.; Goicoechea-Pappas, M.; Anklekar, T. V.; Hart, G. C.; Smith, G. A.; Gawley, R. E., J. Am. Chem. Soc. 1989, 111, 2211.
- 74 Meyers, A. I.; Elworthy, T. R., J. Org. Chem. 1992, 57, 4732.
- 75 Meyers, A. I.; Dickman, D. I.; Bailey, T. R., J. Am. Chem. Soc. 1985, 107, 7974.
- 76 Gawley, R. E.; Rein, K.; Chemburkar, S. R., J. Org. Chem. 1989, 54, 3002.
- 77 Meyers, A. I., Tetrahedron 1992, 48, 2589.
- 78 Meyers, A. I., Aldrichim. Acta 1985, 18, 59.
- 79 Snieckus, V., C.R. Hebd. Seances Acad. Sci. 1990, 90, 879.
- 80 Slocum, D. W.; Jennings, C. A., J. Org. Chem. 1976, 41, 3653.
- 81 Beak, P.; Snieckus, V., Acc. Chem. Res. 1982, 15, 306.
- 82 (a) Ludt, R. E.; Griffiths, T. S.; McGrath, K. N.; Hauser, C. R., J. Org. Chem. 1973, 38, 1668. (b) Beak, P.; Brown, R. A., J. Org. Chem. 1982, 47, 34.
- 83 Newman, M. S.; Kanakarajan, J., J. Org. Chem. 1980, 45, 2301.
- 84 Winkle, M. R.; Ronald, R. C., J. Org. Chem. 1982, 47, 2101.
- 85 Meyers, A. I.; Lutomski, K., J. Org. Chem. 1979, 44, 4464.
- 86 Ziegler, F. E.; Fowler, K. W., J. Org. Chem. 1976, 41, 1564.
- 87 Reuman, M.; Meyers, A. I., Tetrahedron 1985, 41, 837.
- 88 Furlano, D. C.; Calderon, S. N.; Chen, G.; Kirk, K. L., J. Org. Chem. 1988, 53, 3145.
- 89 Baldwin, J. E.; Bair, K. W., Tetrahedron Lett. 1978, 2559.
- 90 Fuhrer, W.; Gschwend, H. W., J. Org. Chem. 1979, 44, 1133.
- 91
Katsuura, K.;
Snieckus, V.,
Can. J. Chem.
1987,
65,
3165.
10.1139/v87-020 Google Scholar
- 92 Turner, J. A., J. Org. Chem. 1983, 48, 3401.
- 93 Moorhoff, C. M.; Paquette, L. A., J. Org. Chem. 1991, 56, 703.
- 94 Gay, R. L.; Hauser, C. R., J. Am. Chem. Soc. 1967, 89, 1647.
- 95 Adam, W.; Cueto, O., J. Org. Chem. 1977, 42, 38.
- 96 Hosomi, A.; Araki, Y.; Sakurai, H., J. Am. Chem. Soc. 1982, 104, 2081.
- 97 Meyers, A. I.; Malone, G. R.; Adickes, H. W., Tetrahedron Lett. 1970, 3715.
- 98 (a) Sauvetre, R.; Roux-Schmitt, M.-C.; Seyden-Penne, J., Tetrahedron Lett. 1978, 34, 2135. (b) Sauvetre, R.; Seyden-Penne, J., Tetrahedron Lett. 1976, 3949.
- 99 Takano, S.; Shimazaki, Y.; Takahashi, M.; Ogasawara, K., J. Chem. Soc., Chem. Commun. 1988, 1004.
- 100 Tamaru, Y.; Kagotini, M.; Furukawa, Y.; Amino, Y.; Yoshida, Z., Tetrahedron Lett. 1981, 22, 3413.
- 101 Hucklin, S. N.; Weiler, L., J. Am. Chem. Soc. 1974, 96, 4691.
- 102 Fraser, R. R.; Banville, J.; Dhawan, K. C., J. Am. Chem. Soc. 1978, 100, 7999.
- 103 Kanemasa, S.; Tatsukawa, A.; Wada, E., J. Org. Chem. 1991, 56, 2875.
- 104 Laborde, E.; Kiely, J. S.; Culbertson, T. P.; Leheski, L. E., J. Med. Chem. 1993, 36, 1964.
- 105 Cahiez, G.; Bernard, D.; Normant, J. F., Synthesis 1976, 245.
- 106 Kitatani, K.; Hiyama, T.; Nozaki, H., J. Am. Chem. Soc. 1975, 97, 949.
- 107 Hoeg, D. F.; Lusk, D. I.; Crumbliss, A. L., J. Am. Chem. Soc. 1965, 87, 4147.
- 108
Foulger, N. J.;
Wakefield, B. J.,
Organomet. Synth.
1986,
3,
369.
10.1016/B978-0-444-42607-9.50098-X Google Scholar
- 109 Kitatani, K.; Hiyama, T.; Nozaki, H., Bull. Chem. Soc. Jpn. 1977, 50, 3288.
- 110 Georgoulis, C.; Meyet, J.; Smajda, W., J. Organomet. Chem. 1976, 121, 271.
- 111 Panek, E. J.; Neff, B. L.; Chu, H.; Panek, M. G., J. Am. Chem. Soc. 1975, 97, 3996.
- 112 Cooke, Jr., M. P., J. Org. Chem. 1993, 58, 2910.
- 113 Bailey, W. F.; Nurmi, T. T.; Patricia, J. J.; Wang, W., J. Am. Chem. Soc. 1987, 109, 2442.
- 114 (a) Bailey, W. F.; Punzalan, E. P., J. Org. Chem. 1990, 55, 5404. (b) Negishi, E.; Swanson, D. R.; Rousset, C. J., J. Org. Chem. 1990, 55, 5406.
- 115
(a)
Peterson, D. J.;
Ward, J. F.,
J. Organomet. Chem.
1974,
66,
209.
(b)
Seyferth, D.;
Mammarella, R. E.,
J. Organomet. Chem.
1979,
77,
53.
10.1016/S0022-328X(00)92331-3 Google Scholar
- 116 (a) Still, W. C.; Sreekumar, C., J. Am. Chem. Soc. 1980, 102, 1201. (b) Sawyer, J. S.; Kucerovy, A.; Macdonald, T. L.; McGarvey, G. J., J. Am. Chem. Soc. 1988, 110, 842. (c) Sawyer, J. S.; Macdonald, T. L.; McGarvey, G. J., J. Am. Chem. Soc. 1984, 106, 3376.
- 117 Lohse, P.; Loner, H.; Acklin, P.; Sternfeld, F.; Pfaltz, A., Tetrahedron Lett. 1991, 32, 615.
- 118 (a) Chong, J. M.; Park, S. B., J. Org. Chem. 1992, 57, 2220. (b) Pearson, W. H.; Lindbeck, A. C., J. Org. Chem. 1989, 54, 5651. (c) Pearson, W. H.; Lindbeck, A. C., J. Am. Chem. Soc. 1991, 113, 8546. (d) N-stannylmethanimines: Pearson, W. H.; Szura, D. P.; Postich, M. J., J. Am. Chem. Soc. 1992, 114, 1329.
- 119 Chan, P. C.-M.; Chong, J. M., Tetrahedron Lett. 1990, 31, 1985.
- 120 Broka, C. A.; Shen, T., J. Am. Chem. Soc. 1989, 111, 2981.
- 121
(a)
McGarvey, G. J.;
Kimura, M.,
J. Org. Chem.
1985,
50,
4652.
10.1021/jo00223a051 Google Scholar(b) Krief, A.; Hobe, M., Tetrahedron Lett. 1992, 33, 6527.
- 122 Krief, A.; Evrard, G.; Badaoui, E.; DeBeys, V.; Dieden, R., Tetrahedron Lett. 1989, 30, 5635.
- 123 Krief, A.; Dumont, W.; Clarembeau, M.; Bernard, G.; Badaoui, E., Tetrahedron 1989, 45, 2005.
- 124 Reich, H. J.; Bowe, M. D., J. Am. Chem. Soc. 1990, 112, 8994.
- 125 (a) Reich, H. J., Acc. Chem. Res. 1979, 12, 22. (b) Liotta, D., Acc. Chem. Res. 1984, 17, 28. (c) Clive, D. L. J., Tetrahedron 1978, 34, 1049. (d) Davis, F. A.; Reddy, R. T., J. Org. Chem. 1992, 2599.
- 126 Seebach, D.; Beck, A. K., Angew. Chem., Int. Ed. Engl. 1974, 13, 806.
- 127
Tomoki, H.;
Kambe, N.;
Ogawa, A.;
Miyoshi, N.;
Murai, S.;
Sonoda, N.,
Angew. Chem., Int. Ed. Engl.
1987,
26,
1187.
10.1002/anie.198711871 Google Scholar
- 128 Curtin, D. Y.; Koehl, Jr., W. J., J. Am. Chem. Soc. 1962, 84, 1967.
- 129
Marshall, J. A.,
Comprehensive Organic Synthesis
1991,
3,
975.
10.1016/B978-0-08-052349-1.00087-1 Google Scholar
- 130 Crombie, L.; Darnbrough, G.; Pattenden, G., J. Chem. Soc., Chem. Commun. 1976, 684.
- 131 Midland, M. M.; Kwon, Y. C., Tetrahedron Lett. 1985, 26, 5021.
- 132 Tsai, D. J.-S.; Midland, M. M., J. Org. Chem. 1984, 49, 1842.
- 133 Nakai, T.; Mikami, K.; Taya, S.; Fujita, Y., J. Am. Chem. Soc. 1981, 103, 6492.
- 134
(a)
Marshall, J. A.;
Robinson, E. D.;
Lebreton, J.,
J. Org. Chem.
1990,
55,
227.
(b)
Takashi, T.;
Nemoto, H.;
Kanda, Y.;
Tsuji, J.;
Fujise, Y.,
J. Org. Chem.
1986,
51,
4315.
10.1021/jo00372a047 Google Scholar
- 135 Bulman Page, P. C.; Rayner, C. M.; Sutherland, I. O., J. Chem. Soc., Chem. Commun. 1988, 356.
- 136 Kuwajima, I.; Kato, M., Tetrahedron Lett. 1980, 21, 623.
- 137 Klein, K. P.; Van Eenam, D. N.; Hauser, C. R., J. Org. Chem. 1967, 32, 1155.
- 138 Harvey, R. G.; Cho, H., J. Am. Chem. Soc. 1974, 96, 2434.
- 139 Hunt, E.; Lythgoe, B., J. Chem. Soc., Chem. Commun. 1972, 757.
- 140 Gilman, H.; Gorsich, R. D., J. Am. Chem. Soc. 1957, 79, 2625.
- 141 Fischer, P.; Schaefer, G., Angew. Chem., Int. Ed. Engl. 1981, 20, 863.
- 142 Cainelli, G.; Ronchi, A. U.; Bertini, F.; Grasselli, P.; Zubiani, G., Tetrahedron 1971, 27, 6109.
- 143 Bandouy, R.; Gore, J.; Ruest, L., Tetrahedron 1987, 43, 1099.
- 144 Corey, E. J.; Fuchs, P. L., Tetrahedron Lett. 1972, 3769.
- 145 Romo, D.; Johnson, D. D.; Plamondon, L.; Miwa, T.; Schreiber, S. L., J. Org. Chem. 1992, 57, 5060.
- 146
Schone, N. E.;
Knudsen, M. J.,
J. Org. Chem.
1987,
52,
569.
10.1021/jo00380a017 Google Scholar
- 147 Chamberlin, A. R.; Bond, F. T., Synthesis 1979, 44.
- 148
Tomooka, K.;
Yamamoto, H.;
Nakai, T.,
Angew. Chem. Int. Ed.
2000,
39,
4500.
10.1002/1521-3773(20001215)39:24<4500::AID-ANIE4500>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 149
Tomooka, K.;
Kikuchi, M.;
Igawa, K.;
Suzuki, M.;
Keong, P.-H.;
Nakai, T.,
Angew. Chem. Int. Ed.
2000,
39,
4502.
10.1002/1521-3773(20001215)39:24<4502::AID-ANIE4502>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 150 Schaudt, M.; Blechert, S., J. Org. Chem. 2003, 68, 2913.
- 151 Lampe, J. W.; Hughes, P. F.; Biggers, C. K.; Smith, S. H.; Hu, H., J. Org. Chem. 1996, 61, 4572.
- 152 Reed, M. A.; Chang, M. T.; Snieckus, V., Org. Lett. 2004, 6, 2297.
- 153 Stará, I. G.; Starý, I.; Tichý, M.; Závada, J.; Hanus, V., J. Am. Chem. Soc. 1994, 116, 5084.
- 154 Norsikian, S.; Marek, I.; Normant, J. F., Tetrahedron Lett. 1997, 38, 7523.
- 155 Norsikian, S.; Marek, I.; Klein, S.; Poisson, J. F.; Normant, J. F., Chem. Eur. J. 1995, 5, 2055.
- 156 Hogan, L. A.-M.; O'Shea, D. F., J. Am. Chem. Soc. 2006, 128, 10360.
- 157 Hogan, L. A.-M.; O'Shea, D. F., J. Org. Chem. 2008, 73, 2503.
- 158 Hogan, L. A.-M.; Tricotet, T.; Meek, A.; Khokhar, S. S.; O'Shea, D. F., J. Org. Chem. 2008, 73, 6041.
- 159 Hogan, A.-M. L.; O'Shea, D. F., J. Am. Chem. Soc. 2006, 128, 10360.
- 160 Hogan, A.-M. L.; O'Shea, D. F., J. Org. Chem. 2008, 73, 2503.
- 161 Ona-Burgos, P., Fernández, I.; Roces, L.; Torre-Fernández, L.; Gracía-Granada, S.; López-Ortiz, F., Org. Lett. 2008, 10, 3195.
- 162 Ona-Burgos, P., Fernández, I.; Iglesias, M. J.; Gracía-Granada, S.; López-Ortiz, F., Org. Lett. 2008, 10, 537.
- 163 Hoarau, C.; Couture, A.; Deniau, E.; Grandclaudon, P., J. Org. Chem. 2002, 67, 5846.
- 164 Malkov, A. V.; Bella, M.; Stará, I. G.; Kocovský, P., Tetrahedron Lett. 2001, 42, 3045.
- 165 Derdau, V.; Snieckus, V., J. Org. Chem. 2001, 66, 1992.
- 166 MacNeil, S. L.; Familoni, O. B.; Snieckus, S., J. Org. Chem. 2001, 66, 3662.
- 167 Macklin, T. K.; Reed, M. A.; Snieckus, V., Eur. J. Org. Chem. 2008, 9, 1507.
- 168 Proudfoot, J. R.; Patel, U. R.; Dyatkin, A. B., J. Org. Chem. 1997, 62, 1851.
- 169 Metallinos, C.; Nerdinger, S.; Snieckus, V., Org. Lett. 1999, 1, 1183.
- 170 Blanchet, J.; Macklin, T.; Ang, P.; Metallinos, C.; Snieckus, V., J. Org. Chem. 2007, 72, 3199.
- 171 Kauch, M.; Hoppe, D., Can. J. Chem. 2001, 79, 1736.
- 172 Kauch, M.; Snieckus, V.; Hoppe, D., J. Org. Chem. 2005, 70, 7149.
- 173 Nerdinger, S.; Kendall, C.; Cai, X.; Marchart, R.; Riebel, P.; Johnson, M. R.; Yin, C.-F.; Hénaff, N.; Eltis, L. D.; Snieckus, V., J. Org. Chem. 2007, 72, 5960.
- 174 Srinivasan, K.; Laughrey, Z. R.; Gibb, B. C., Eur. J. Org. Chem. 2008, 3265.
- 175 Gu, Y. G.; Bayburt, E. K.; Michaelides, M. R.; Lin, C. W.; Shiosaki, K., Bioorg. Med. Chem. Lett. 1999, 9, 1341.
- 176 Suffert, J.; Toussaint, D., J. Org. Chem. 1995, 60, 3550.
- 177 Macklin, T. K.; Snieckus, V., Org. Lett. 2005, 7, 2519.
- 178 Dyke, A. M.; Gill, D. M.; Harvey, J. N.; Hester, A. J.; Lloyd-Jones, G. C.; Munoz, M. P.; Shepperson, I. R., Angew. Chem. Int. Ed. 2008, 47, 5067.
- 179 McCubbin, J. A.; Tong, X.; Wang, R.; Zhao, Y.; Snieckus, V.; Lemieux, R. P., J. Am. Chem. Soc. 2004, 126, 1161.
- 180 Takaya, Y.; Ogasawara, M.; Hayashi, T., Tetrahedron Lett. 1999, 40, 6957.
- 181
Skai, M.;
Hayashi, H.;
Miyaura, N.,
Organometallics
1997,
16,
4229.
10.1021/om9705113 Google Scholar
- 182 Hayashi, T.; Yamasaki, K., Chem. Rev. 2003, 103, 2829.
- 183 Billingsley, K. L.; Buchwald, S. L., Angew. Chem. Int. Ed. 2008, 47, 4695.
- 184 Dondoni, A.; Marra, A.; Mizuno, M.; Giovannini, P. P., J. Org. Chem. 2002, 67, 4186.
- 185 Sekine, A.; Ohshima, T.; Shibasaki, M., Tetrahedron 2002, 58, 75.
- 186 Smith, A. B., III; Minbiole, K. P.; Verhoest, P. R.; Schelhaas, M., J. Am. Chem. Soc. 2001, 123, 10942.
- 187 Gómez, A. M.; Casillas, M.; Barrio, A.; Gawel, A.; López, J. C., Eur. J. Org. Chem. 2008, 3933.
- 188 Michel, P.; Gennet, D.; Rassat, A., Tetrahedron Lett. 1999, 40, 8575.
- 189 Denmark, S. E.; Yang, S.-M., J. Am. Chem. Soc. 2002, 124, 15196.
- 190 Toro, A.; Nowak, P.; Deslongchamps, P., J. Am. Soc. Chem. 2000, 122, 4526.
- 191 Tsukazaki, M.; Tinkl, M.; Roglans, A.; Chapell, B. J.; Taylor, N. J.; Snieckus, V., J. Am. Chem. Soc. 1996, 118, 685.
- 192 Laufer, R. S.; Veith, U.; Taylor, N. J.; Snieckus, V., Org. Lett. 2000, 2, 629.
- 193 Metallinos, C.; Snieckus, V., Org. Lett. 2004, 4, 1935.
- 194 Miao, B.; Tinkl, M.; Snieckus, V., Tetrahedron Lett. 1999, 40, 2449.
- 195 Smith, A. B., III; Kanoh, N.; Ishiyama, H.; Minakawa, N.; Rainier, J. D.; Hartz, R. A.; Cho, Y. S.; Cui, H., Moser, W. H., J. Am. Chem. Soc. 2003, 125, 8228.
- 196 Node, M.; Hashimoto, D.; Katoh, T.; Ochi, S.; Ozeki, M.; Watanabe, T.; Kajimoto, T., Org. Lett. 2008, 10, 2653.
- 197 Molander, G. A.; St. Jean, D. J., Jr.; Haas, J., J. Am. Chem. Soc. 2004, 126, 1642.
- 198 Sharpless, K. B.; Umbreit, M. A.; Nieth, M. T.; Flood, T. C., J. Am. Chem. Soc. 1972, 94, 6538.
- 199 Johnson, J.; Kim, S.-H.; Bifano, M.; DiMarco, J.; Fairchild, C.; Gougoutas, J.; Lee, F.; Long, B.; Tokarski, J.; Vite, G., Org. Lett. 2000, 2, 1537.
- 200 Palacios, F.; Ochoa de Retana, A.; Oyarzabal, J.; Pascual, S.; Fernández de Trocóniz, G., J. Org. Chem. 2008, 73, 4568.
- 201 Duguet, N.; Petit, S. M.; Marchand, P.; Harrison-Marchand, A.; Maddaluno, J., J. Org. Chem. 2008, 73, 5397.



