Abstract
[7439-93-2] Li (MW 6.94)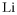
InChI = 1S/Li
InChIKey = WHXSMMKQMYFTQS-UHFFFAOYSA-N
(powerful reducing agent;1 used for partial reduction of aromatics and conjugated polyenes;1e,m–o conversion of alkynes to trans-alkenes;1a,h stereoselective reduction of hindered ketones;11 enone reduction and regioselective alkylation;1f,j reductive cleavage of polar single bonds1a)
Physical Data: mp 180.5 °C; bp 1327 ± 10 °C; d 0.534 g cm−3. Natural isotopic composition: 7Li (92.6 %); 6Li (7.4 %).
Solubility: 10.9 g/100 g NH3 at −33 °C (= 74.1 g/L NH3); 36.5 g/L MeNH2 at −23 °C.
Form Supplied in: under Ar, as solid in the form of wire, ribbon, rod, foil, shot, ingot, or as a powder; in mineral oil, as wire, shot, or as 25–30 wt % dispersions.
Purify: commercially available in up to 99.97% purity. In general, lithium is not further purified except for cutting off the surface coating.
Handling, Storage, and Precautions: best stored under mineral oil in airtight steel drums and handled under Ar or He. Dispersions in mineral oil segregate on storage and uniformity is restored by stirring. The mineral oil is washed off under Ar with pentane or hexane, and the metal is either dried in an Ar stream or rinsed with the reaction solvent. Dry Li powder is extremely reactive towards air, H2O vapor, and N2. The metal reacts rapidly with moist air at 25 °C, but with dry air or dry O2 only at higher temperatures (>100 °C). A slight blow can initiate violent burning. Reaction with N2 already occurs at 25 °C, but is inhibited by traces of O2. Li reacts readily with H2O, but does not spontaneously ignite as the other alkali metals do. It reacts rapidly with dil HCl and H2SO4 and vigorously with HNO3. Ready reaction occurs with halogens.
References
- 1
(a)
Smith, M., In
Reduction: Techniques and Applications in Organic Synthesis;
R. L. Augustine, Ed.;
Dekker:
New York,
1968;
Chapter 2.
(b)
Hudlickyì, M.,
Reductions in Organic Chemistry;
Horwood:
Chichester,
1984.
(c)
Birch, A. J.,
Q. Rev., Chem. Soc.
1950,
4,
69.
(d)
Benkeser, R. A.,
Adv. Chem. Ser.
1957,
23,
58.
10.1021/ba-1959-0023.ch005 Google Scholar(e) Birch, A. J.; Smith, H., Q. Rev., Chem. Soc. 1958, 12, 17. (f) Steroid Reactions; C. Djerassi, Ed.; Holden-Day: San Francisco, 1963. (g) Harvey, R. G., Synthesis 1970, 161. (h) Kaiser, E. M., Synthesis 1972, 391. (i) Birch, A. J.; Subba Rao, G. S. R., Adv. Org. Chem. 1972, 8, 1. (j) Caine, D., Org. React. 1976, 23, 1. (k) Brendel, G., Lithium Metal in Organic Synthesis; In 3rd Lect.-Hydride Symp.; Metallges. AG: Frankfurt/Main, 1979; p 135. (1) Huffman, J. W., Acc. Chem. Res. 1983, 16, 399. (m) Hook, J. M.; Mander, L. N., Nat. Prod. Rep. 1986, 3, 35. (n) Rabideau, P. W., Tetrahedron 1989, 45, 1579. (o) Rabideau, P. W.; Marcinow, Z., Org. React. 1992, 42, 1.
- 2 (a) Dye, J. L., Prog. Inorg. Chem. 1984, 32, 327. (b) Thompson, J. C., Monographs on the Physics and Chemistry of Materials: Electrons in Liquid Ammonia; Oxford University Press: Fair Lawn, N. J.; 1976.
- 3 Gremmo, N.; Randles, J. E. B., J. Chem. Soc., Faraday Trans. 1 1974, 70, 1480.
- 4 (a) Dewald, R. R., J. Prakt. Chem. 1975, 79, 3044. (b) Birch, A. J.; Slobbe, J., Heterocycles 1976, 5, 905.
- 5 (a) Pleskov, V. A., J. Phys. Chem. (U.S.S.R) 1937, 9, 12; (Chem. Abstr. 1937, 31, 4214). (b) Wilds, A. L.; Nelson, N. A., J. Am. Chem. Soc. 1953, 75, 5360.
- 6 Johnson, W. C.; Piskur, M. M., J. Prakt. Chem. 1933, 37, 93.
- 7For reductions with lithium bronze, see (a) Mueller, R. H.; Gillick, J. G., J. Org. Chem. 1978, 43, 4647. (b) Fang, J.-M., J. Org. Chem. 1982, 47, 3464.
- 8 Krapcho, A. P.; Bothner-By, A. A., J. Am. Chem. Soc. 1959, 81, 3658.
- 9 Dryden, H. L., Jr.; Webber, G. M.; Burtner, R. R.; Cella, J. A., J. Org. Chem. 1961, 26, 3237.
- 10 Evers, E. C.; Young, A. E., II; Panson, A. J., J. Am. Chem. Soc. 1957, 79, 5118.
- 11 Dewald, R. R.; Dye, J. L., J. Prakt. Chem. 1964, 68, 128.
- 12 Benkeser, R. A.; Belmonte, F. G.; Kang, J., J. Org. Chem. 1983, 48, 2796.
- 13
Mander, L. N.,
Comprehensive Organic Synthesis
1991,
8,
489.
10.1016/B978-0-08-052349-1.00237-7 Google Scholar
- 14 Turner, R. B.; Gänshirt, K. H.; Shaw, P. E.; Tauber, J. D., J. Am. Chem. Soc. 1966, 88, 1776.
- 15 Fried, J.; Abraham, N. A., Tetrahedron Lett. 1965, 3505.
- 16 Fried, J.; Abraham, N. A.; Santhanakrishnan, T. S., J. Am. Chem. Soc. 1967, 89, 1044.
- 17 Camps, F.; Coll, J.; Pascual, J., J. Org. Chem. 1967, 32, 2563.
- 18 Baker, A. J.; Goudie, A. C., J. Chem. soc., Chem. Commun. 1972, 951.
- 19 Sipio, W. J., Tetrahedron Lett. 1985, 26, 2039.
- 20 Subba Rao, G. S. R.; Ramanathan, H.; Raj, K., J. Chem. soc., Chem. Commun. 1980, 315.
- 21 Hamilton, R. J.; Mander, L. N.; Sethi, S. P., Tetrahedron 1986, 42, 2881.
- 22 Schultz, A. G.; Sundararaman, P.; Macielag, M.; Lavieri, F. P.; Welch, M., Tetrahedron Lett. 1985, 26, 4575.
- 23 Rabideau, P. W.; Karrick, G. L., Tetrahedron Lett. 1987, 28, 2481.
- 24 Burgstahler, A. W.; Worden, L. R., J. Am. Chem. Soc. 1964, 86, 96.
- 25 Borowitz, I. J.; Gonis, G.; Kelsey, R.; Rapp, R.; Williams, G. J., J. Org. Chem. 1966, 31, 3032.
- 26 Reggel, L.; Friedel, R. A.; Wender, I., J. Org. Chem. 1957, 22, 891.
- 27 Rabideau, P. W.; Burkholder, E. G., J. Org. Chem. 1978, 43, 4283.
- 28 (a) Jaworek, W.; Vögtle, F., Chem. Ber. 1991, 124, 347; (Chem. Abstr. 1991, 114, 101 319p). (b) Michel, P.; Moradpour, A., Synthesis 1988, 894.
- 29 (a) Koch, K.-H.; Müllen, K., Chem. Ber. 1991, 124, 2091. (b) Anton, U.; Göltner, C.; Müllen, K., Chem. Ber. 1992, 125, 2325.
- 30 Julina, R.; Herzig, T.; Bernet, B.; Vasella, A., Helv. Chim. Acta 1986, 69, 368.
- 31 Dear, R. E. A.; Pattison, F. L. M., J. Am. Chem. Soc. 1963, 85, 622.
- 32 Svoboda, M.; Závada, J.; Sicher, J., Collect. Czech Chem. Commun. 1965, 30, 413.
- 33 (a) Stork, G.; Malhotra, S.; Thompson, H.; Uchibayashi, M., J. Am. Chem. Soc. 1965, 87, 1148. (b) Miller, B. R., Synth. Commun. 1972, 2, 273.
- 34 (a) Stork, G.; Boeckmann, R. K., Jr.; Taber, D. F.; Still, W. C.; Singh, J., J. Am. Chem. Soc. 1979, 101, 7107. (b) Swartz, J. E.; Mahachi, T. J.; Kariv-Miller, E., J. Am. Chem. Soc. 1988, 110, 3622.
- 35
Huffman, J. W.,
Comprehensive Organic Synthesis
1991,
8,
107.
10.1016/B978-0-08-052349-1.00220-1 Google Scholar
- 36 Huffman, J. W.; Desai, R. C.; LaPrade, J. E., J. Org. Chem. 1983, 48, 1474.
- 37 Giroud, A. M., Rassat, A., Bull. Soc. Chem. Fr. 1976, 1881; (Chem. Abstr. 1977, 87, 6251a).
- 38 Toromanoff, E., Bull. Soc. Chem. Fr. 1987, 893; (Chem. Abstr. 1988, 109, 128 193b).
- 39 (a) Stork, G.; Rosen, P.; Goldman, N.; Coombs, R. V.; Tsuji, J., J. Am. Chem. Soc. 1965, 87, 275. (b) Stork, G.; Logusch, E. W., J. Am. Chem. Soc. 1980, 102, 1218.
- 40 Samson, M.; De Clercq, P.; Vandewalle, M., Tetrahedron 1977, 33, 249.
- 41 (a) Burgstahler, A. W.; Sanders, M. E., Synthesis 1980, 400. (b) See also Fétizon, M.; Gore, J., Tetrahedron Lett. 1966, 471.
- 42 Ireland, R. E.; Pfister, G., Tetrahedron Lett. 1969, 2145.
- 43 (a) Hall, S. S.; Lipsky, S. D.; McEnroe, F. J.; Bartels, A. P., J. Org. Chem. 1971, 36, 2588. (b) Marcinow, Z.; Rabideau, P. W., J. Org. Chem. 1988, 53, 2117.
- 44 (a) Hall, S. S.; Lipsky, S. D., J. Org. Chem. 1973, 38, 1735. (b) For phenylation–reduction of aliphatic ketones see Hall, S. S.; McEnroe, F. J., J. Org. Chem. 1975, 40, 271.
- 45 Bedenbaugh, A. O.; Bedenbaugh, J. H.; Bergin, W. A.; Adkins, J. D., J. Am. Chem. Soc. 1970, 92, 5774.
- 46 (a) Bruck, P.; Thompson, D.; Winstein, S., Chem. Ind. (London) 1960, 405. (b) Ikan, R.; Markus, A., J. Chem. Soc., Perkin Trans. 1 1972, 2423. (c) Bruck, P., Tetrahedron Lett. 1962, 449. (d) For substitution of Li by Na, see: Gassman, P. G.; Pape, P. G., J. Org. Chem. 1964, 29, 160.
- 47 (a) Pinder, A. R., Synthesis 1980, 425. (b) Duggan, A. J.; Hall, S. S., J. Org. Chem. 1975, 40, 2238. (c) Berkowitz, D. B., Synthesis 1990, 649.
- 48 (a) Ziegler, K.; Colonius, H., Justus Liebigs Ann. Chem. 1930, 479, 135; (Chem. Abstr. 1930, 24, 3777). (b) Jones, R. G.; Gilman, H., Org. React. 1951, 6, 339.
- 49 Han, B. H.; Boudjouk, P., Tetrahedron Lett. 1981, 22, 2757.
- 50
Caubère, P.;
Coutrot, P.,
Comprehensive Organic Synthesis
1991,
8,
835.
10.1016/B978-0-08-052349-1.00248-1 Google Scholar
- 51 (a) Ohmori, M.; Yamada, S.; Takayama, H., Tetrahedron Lett. 1982, 23. (b) Grieco, P. A.; Masaki, Y., J. Org. Chem. 1974, 39, 2135. (c) Stotter, P. L.; Hornish, R. E., J. Am. Chem. Soc. 1973, 95, 4444. See also Truce, W. E.; Tate, D. P.; Burdge, D. N., J. Am. Chem. Soc. 1960, 82, 2872.
- 52 Sevrin, M.; Van Ende, D.; Krief, A., Tetrahedron Lett. 1976, 2643.
- 53 Brown, H. C.; Ikegami, S.; Kawakami, J. H., J. Org. Chem. 1970, 35, 3243.
- 54 (a) Markgraf, J. H.; Hensley, W. M.; Shoer, L. I., J. Org. Chem. 1974, 39, 3168. (b) Boar, R. B.; Joukhadar, L.; McGhie, J. F.; Misra, S. C., J. Chem. soc., Chem. Commun. 1978, 68.
- 55 Staley, S. W., Sel. Org. Transform. 1972, 2, 309.
- 56 White, J. D., Tetrahedron Lett. 1974, 2879.
- 57 Cuvigny, T.; Larchevêque, M., J. Organometallic Chem. 1974, 64, 315; (Chem. Abstr. 1974, 80, 70 494q).
- 58 McMurry, J. E.; Fleming, M. P.; Kees, K. L.; Krepski, L. R., J. Org. Chem. 1978, 43, 3255.
- 59 Yus, M.; Radivoy, G.; Alonso, F., Synthesis 2001, 3, 427.
- 60 Yus, M.; Radivoy, G.; Alonso, F., Tetrahedron 2000, 56, 8673.
- 61 Yadav, J. S.; Prahlad, V.; Chander, M., J. Chem. Soc., Chem. Commun. 1993, 137.
- 62 Yus, M.; Herrera, R.; Guijarro, A., Tetrahedron Lett. 2003, 44, 1313.
- 63 Fry, J. L.; Karaman, R.; Kohlman, D. T., Tetrahedron Lett. 1990, 31, 6155.
- 64 Luche, J. L.; Aurell, M. J.; Danhui, Y., Synlett 1995, 459.
- 65 Luche, J. L.; Aurell, M. J.; Danhui, Y.; Einhorn, J.; Einhorn, C., J. Chem. Soc., Chem. Commun. 1994, 1815.
- 66 Welch, J. T.; Gyenes, F.; Bergman, K., J. Org. Chem. 1998, 63, 2824.
- 67 Azzena, U.; Melloni, G.; Pisano, L.; Sechi, B., Tetrahedron Lett. 1994, 35, 6759.
- 68 Melero, C.; Guijarro, A.; Baumann, V.; Pérez-Jiménez, A.; Yus, M., Eur. J. Org. Chem. 2007, 5514.
- 69 Srikrishna, A.; Ramasastry, S. S. V., Tetrahedron Lett. 2004, 45, 379.
- 70 Schmidt, A. W.; Doert, T.; Goutal, S.; Gruner, M.; Mende, F.; Kurzchalia, T. V.; Knölker, H.-J., Eur. J. Org. Chem. 2006, 3687.
- 71 Nador, F.; Moglie, Y.; Ciolino, A.; Pierini, A.; Dorn, V.; Yus, M.; Alonso, F.; Radivoy, G., Tetrahedron Lett. 2012, 53, 3156.
- 72 Yang, A.; Butela, H.; Deng, K.; Doubleday, M. D.; Cohen, T., Tetrahedron 2006, 62, 6526.
- 73 Azzena, U.; Dettori, G.; Pisano, L.; Pittalis, M.; Mangano, G.; Petretto, G.; Pintore, G., Monatsh. Chem. 2012, 143, 601.
- 74 Azzena, U.; Dettori, G.; Sforazzini, G.; Yus, M.; Foubelo, F., Tetrahedron 2006, 62, 1557.
- 75 Pittalis, M.; Azzena, U.; Pisano, L., Tetrahedron 2013, 69, 207.
- 76 Mother, W. B.; Zuberi, S., Tetrahedron Lett. 2006, 47, 8789.
- 77 Lillo, V. J.; Gómez, C.; Yus, M., ARKIVOC 2010, (iii), 29.



