Allyl Phenyl Sulfoxide†
Abstract
[19093-37-9] C9H10OS (MW 166.23)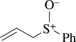
InChI = 1S/C9H10OS/c1-2-8-11(10)9-6-4-3-5-7-9/h2-7H,1,8H2
InChIKey = RLLJHMBTZSGFAS-UHFFFAOYSA-N
(allylic sulfoxides undergo allylic rearrangement to form sulfenate esters, allowing the generation of allylic alcohols; metalated derivatives serve as ambident nucleophiles; precursors to Pummerer intermediates)
Physical Data: bp 103–104 °C/0.36 mmHg; d 1.1205 g cm−3.2
Solubility: insol water; sparingly sol hexane; sol most other organic solvents.
Preparative Methods: oxidation of Allyl Phenyl Sulfide in dichloromethane with m‐Chloroperbenzoic Acid acid (1.2 equiv) added slowly as a solution in dichloromethane at −70 °C affords the sulfoxide in high yields with minimal over-oxidation to the sulfone. The m-chlorobenzoic acid formed may be removed simply by filtration. Other oxidizing agents such as Sodium Bromite3 and Trifluoroperacetic Acid4 afford the sulfoxide in yields greater than 80% and are also highly selective.
Purification: distillation in vacuo prior to use.
Handling, Storage, and Precautions: no special precautions are required. The sulfoxide may be handled normally at the bench for general weighing and transferring. However, for maximum shelf-life it is recommended that it be stored at 4 °C in a sealed vessel with exclusion of oxygen and moisture.



