Journal list menu
Export Citations
Download PDFs
Contents
Review Article
Structurally Diversified Heterocycles and Related Privileged Scaffolds as Potential Urease Inhibitors: A Brief Overview
- Pages: 423-446
- First Published: 28 May 2013
Ureases have been implicated in the pathogenesis of many clinical conditions. Strategies based on urease inhibition are the main treatment of diseases caused by urease-producing bacteria. This review article surveys recent efforts undertaken for the identification, optimization and development of potent urease inhibitors.
Full Papers
Synthesis and Biological Evaluation of Novel Bromophenol Derivatives as Carbonic Anhydrase Inhibitors
- Pages: 447-454
- First Published: 07 May 2013
An alternative synthesis of the natural bromophenol 3,4-dibromo-5-(2,3-dibromo-4,5-dihydroxybenzyl)-6-(ethoxymethyl)benzene-1,2-diol (3) and the first synthesis of (4,5-dihydroxy-2-methylphenyl)(3,4-dihydroxyphenyl)methanone (18) and its brominated derivatives 19–21 are provided. The compounds were tested against two human carbonic anhydrase isozymes, showing strong inhibitory activity against hCA I, but low activity against hCA II.
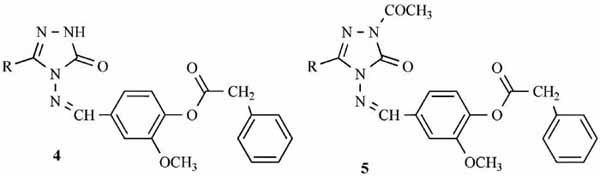
Synthesis and Pharmacological Evaluation of Some Novel Thebaine Derivatives: N-(Tetrazol-1H-5-yl)-6,14-endoethenotetrahydrothebaine Incorporating the 1,3,4-Oxadiazole or the 1,3,4-Thiadiazole Moiety
- Pages: 455-462
- First Published: 03 May 2013
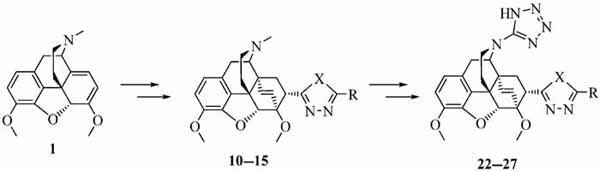
Novel N-(tetrazol-1H-5-yl)-6,14-endoethenotetrahydrothebaine 7α-substituted 1,3,4-oxadiazole and 1,3,4-thiadiazole derivatives were synthesized and their analgesic activity was evaluated by a rat hot-plate test model and a rat tail-flick model. Compound 12 showed analgesic activity higher than that of morphine.
Synthesis and Biological Evaluation of Pyrazoline Derivatives Bearing an Indole Moiety as New Antimicrobial Agents
- Pages: 463-469
- First Published: 16 May 2013
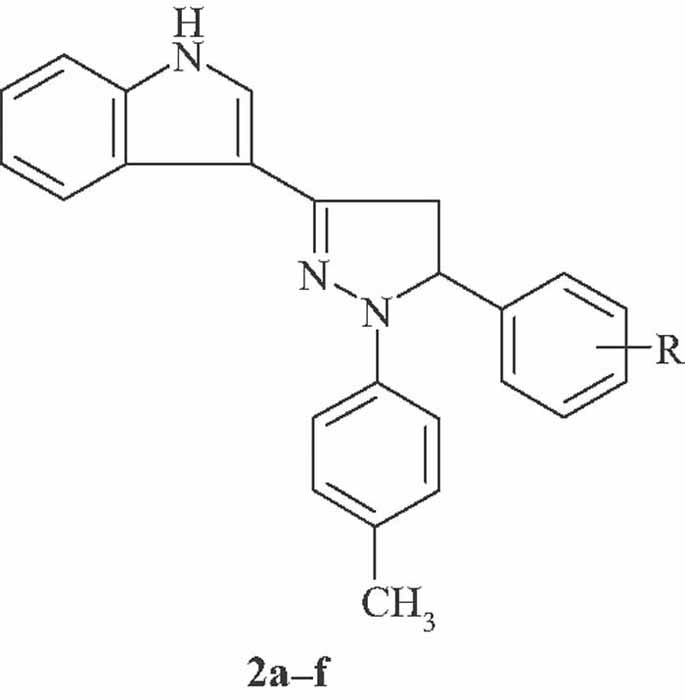
1-(p-Methylphenyl)-3,5-diaryl-2-pyrazoline derivatives (2a–f) synthesized via the treatment of 1-(1H-indol-3-yl)-3-aryl-2-propen-1-ones with p-methylphenylhydrazine hydrochloride in hot acetic acid were investigated for their antimicrobial activity. Compound 2e bearing a 2,5-dichlorophenyl moiety was the most promising agent against Klebsiella pneumoniae and Candida glabrata.
Synthesis, In Vitro Antimicrobial and Antioxidant Activities of Some New 4,5-Dihydro-1H-1,2,4-triazol-5-one Derivatives
- Pages: 470-480
- First Published: 03 May 2013
Synthesis of New N-Substituted 5-Arylidene-2,4-thiazolidinediones as Anti-Inflammatory and Antimicrobial Agents
- Pages: 481-490
- First Published: 13 May 2013
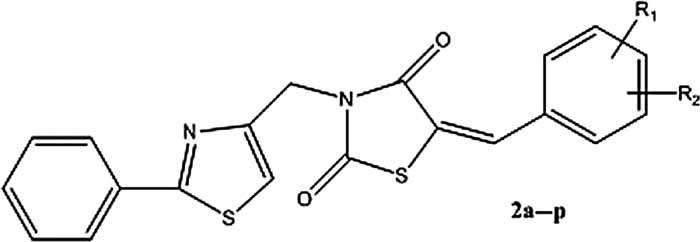
New 5-arylidene-2,4-thiazolidinediones (TZDs) 2a–p were synthesized and evaluated in vivo as anti-inflammatory agents. All except one displayed good results. The phagocytic parameters were decreased by the new molecules. The effect was superior to meloxicam, for the majority of the TZD derivatives. The compounds were also investigated for their antimicrobial properties. Two of them (2e and 2n) manifested growth inhibition capacity on all the tested strains.
Synthesis and Biological Evaluation of 4-Arylphthalazones Bearing Benzenesulfonamide as Anti-Inflammatory and Anti-Cancer Agents
- Pages: 491-498
- First Published: 14 May 2013
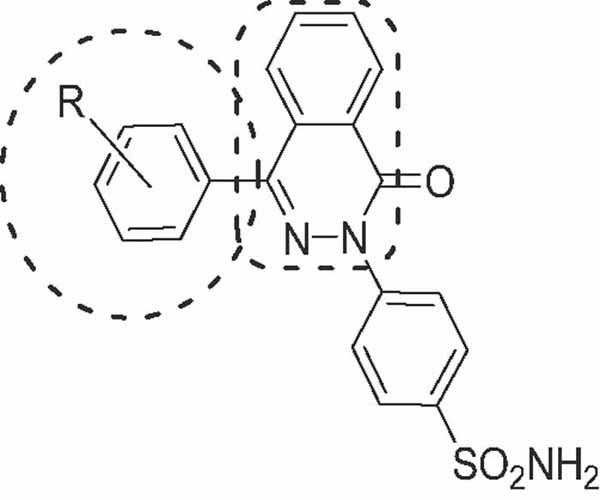
Nine 4-arylphthalazones bearing benzenesulfonamide (2a–i) were synthesized by the condensation of the appropriate 2-aroylbenzoic acid (1a–i) and 4-hydrazinobenzenesulfonamide in ethanol. Compounds 2b and 2i showed anti-inflammatory activity comparable to that of celecoxib in the carrageenan-induced rat paw edema model. Compounds 2d and 2i were screened for their antiproliferative activity towards 60 human cancer cell lines, displaying mild activity toward the renal cancer cell line UO-31.