Journal list menu
Export Citations
Download PDFs
Research Articles
Intrinsically unstructured proteins by design—electrostatic interactions can control binding, folding, and function of a helix-loop-helix heterodimer
- Pages: 461-469
- First Published: 27 June 2013
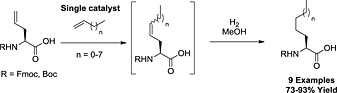
Tandem Ru-alkylidene-catalysed cross metathesis/hydrogenation: synthesis of lipophilic amino acids
- Pages: 470-476
- First Published: 03 June 2013
Distinct solid and solution state self-assembly pathways of RADA16-I designer peptide
- Pages: 477-484
- First Published: 25 June 2013
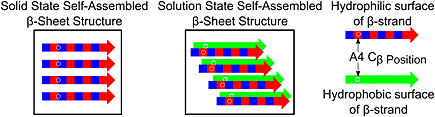
Self-assembly of the RADA16-I peptide in the solid state produces β-sheets that have a structure that differs from those formed through self-assembly in the solution state. Solid state RADA16-I self-assembly produces in-register parallel β-sheets, whereas nanofibers are composed of stacked parallel β-sheets with registry shifts between adjacent β-strands. These results provide evidence for environment-dependent self-assembly mechanisms for RADA16-I β-sheets and new constraints on solid state self-assembled structures that must be avoided to maximize solution solubility and nanofiber yields.
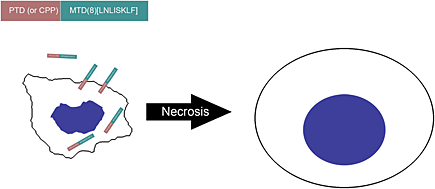
Minimal killing unit of the mitochondrial targeting domain of Noxa
- Pages: 485-490
- First Published: 22 June 2013
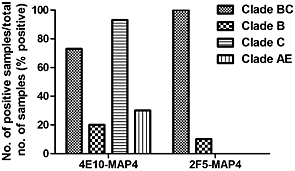
Multiple antigen peptide mimetics containing gp41 membrane-proximal external region elicit broad neutralizing antibodies against human immunodeficiency virus type 1 in guinea pigs
- Pages: 491-498
- First Published: 21 June 2013
Interaction of synthetic peptide octarphin (TPLVTLFK) with human blood lymphocytes
- Pages: 499-503
- First Published: 23 June 2013
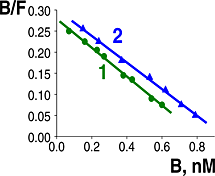
The synthetic peptide octarphin, a selective agonist of non-opioid beta-endorphin receptor, binds with high affinity to human T (Image 1) and B (Image 2) lymphocytes (Kd = 3.0 and 3.2 nM, respectively). The specific binding octarphin labeled with tritium was inhibited by beta-endorphin. The opioid receptor agonists were inactive. Thus, both human blood T and B lymphocytes express non-opioid beta-endorphin receptor.
Recombinant production of TEV cleaved human parathyroid hormone
- Pages: 504-510
- First Published: 23 June 2013
Localization of the anti-cancer peptide EGFR-lytic hybrid peptide in human pancreatic cancer BxPC-3 cells by immunocytochemistry
- Pages: 511-515
- First Published: 01 July 2013
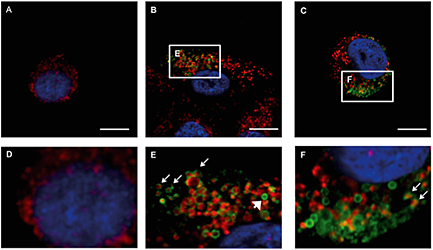
Intracellular localization of EGFR-lytic hybrid peptide in BxPC-3 cells by immunocytochemistry. Green fluorescence of EGFR-lytic peptide was not detected in non-treated cells (A and D), and EGFR-lytic peptide is visible in cytoplasm in the cell treated with EGFR-lytic for 30 min (B and E) and 60 min (C and F). A number of peptides distributed around the red fluorescence of the mitochondria (E and F arrows).
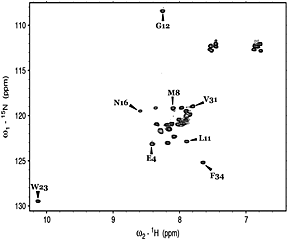
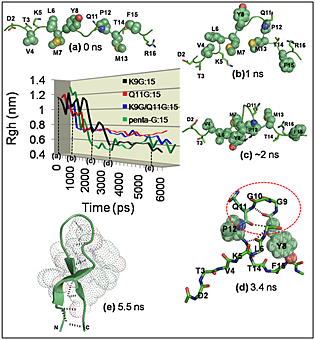
Molecular dynamics simulations of certain mutant peptide models from staphylococcal nuclease reveal that initial hydrophobic collapse associated with turn propensity drives β-hairpin folding
- Pages: 516-527
- First Published: 21 June 2013