Journal list menu
Export Citations
Download PDFs
Articles
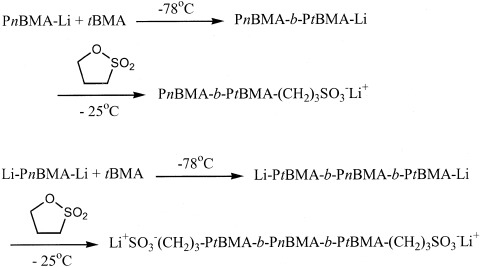
Synthesis of ω- and α,ω-sulfonatotelechelics based on homopolymers and block copolymers of n-butyl methacrylate and t-butyl methacrylate
- Pages: 3711-3721
- First Published: 21 August 2000
Synthesis of photocrosslinkable fluorinated polydimethylsiloxanes: Direct introduction of acrylic pendant groups via hydrosilylation
- Pages: 3722-3728
- First Published: 28 August 2000
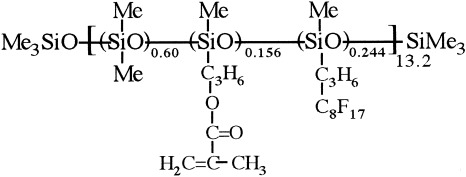
The synthesis of photocrosslinkable fluorinated polydimethylsiloxanes was achieved in two steps through direct hydrosilylation between copoly(dimethyl)(methyl-hydrogen) siloxane, first with a fluorinated olefin and then with allyl methyl methacrylate. The products were characterized by IR, 1H NMR, 19F NMR, and 29Si NMR. The photocrosslinked polymer may find application as a vapor permeation membrane.
Radical ring-opening copolymerization behavior of vinyloxirane: Synthesis of copolymer from 2-isopropenyl-3-phenyloxirane and styrene and its functionalization
- Pages: 3729-3735
- First Published: 28 August 2000
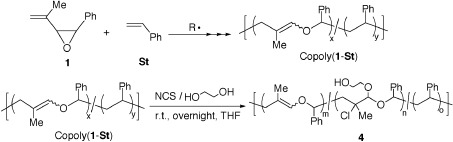
The radical ring-opening copolymerization of 2-isopropenyl-3-phenyloxirane (1) with styrene (St) was examined under various conditions. Copolymers of 1 and St [copoly(1-St)] were obtained in moderate yields by copolymerization in various feed ratios of 1 and St over 120 °C. The haloalkoxylation of copoly(1-St) with ethylene glycol in the presence of N-chlorosuccinimide (NCS) produced a new functional copolymer (4).
Synthesis and characterization of a novel AB2 monomer and corresponding hyperbranched poly(arylene ether phosphine oxide)s
- Pages: 3736-3741
- First Published: 28 August 2000
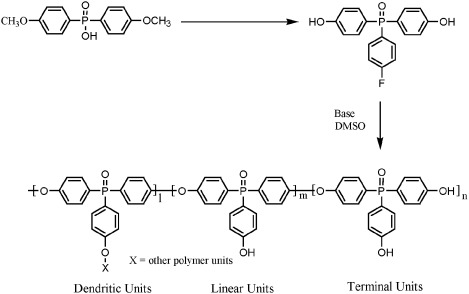
A novel AB2 monomer, 4-(fluorophenyl)-4′,4″-(bishydroxyphenyl) phosphine oxide, was synthesized. The monomer was successfully polymerized to a modest molecular weight with various catalysts, including K2CO3 and Cs2CO3/Mg(OH)2. Hyperbranched polymers exhibited exceptionally high thermal stability and solubility in conventional polar organic solvents and basic water solutions.
Synthesis of central functionalized asymmetric triblock copolymers for surface modification and switchable surface properties
- Pages: 3742-3750
- First Published: 28 August 2000
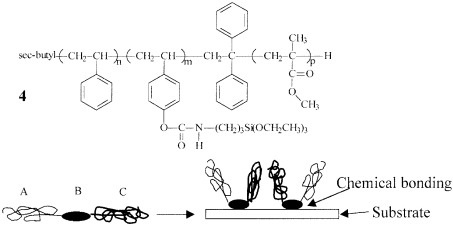
Well-defined central functionalized asymmetric triblock copolymers (CFABC) were designed as a new type of polymer-brush surface modifier with a short central functionalized block that could form chemical bonds with a suitable substrate surface. A combination of sequential living anionic polymerization and polymer modification reactions was used for the synthesis of two CFABCs: polystyrene-b-poly(4-hydroxystyrene)-b-poly(methyl methacrylate) (3) and polystyrene-b-poly(4-urethanopropyl triethoxysilylstyrene)-b-poly(methyl methacrylate) (4). The central block of 3, poly(4-hydroxystyrene), was synthesized with a protected monomer, p-[(tert-butyldimethylsilyl)oxy]styrene, for the polymerization step, and this synthesis was followed by the hydrolysis of the silyl protecting group. To obtain polymer 4, the phenol functionality in 3 was converted to triethoxysilyl groups by a quantitative reaction with isocyanato propyl triethoxysilane. Gel permeation chromatography and NMR characterization indicated that the block copolymers possessed controlled molecular weights and narrow molecular weight distributions. Preliminary atomic force microscopy and X-ray photoelectron spectroscopy analysis of the polymer brushes were reported.
Self-polyaddition of hydroxyalkyl vinyl ethers
- Pages: 3751-3760
- First Published: 30 August 2000
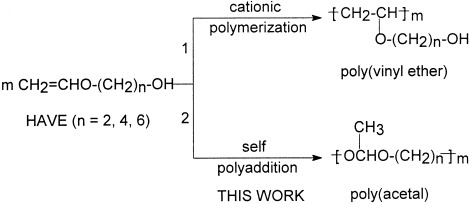
The hydroxyalkyl vinyl ethers 2-hydroxyethyl vinyl ether, 4-hydroxybutyl vinyl ether, and 6-hydroxyhexyl vinyl ether were polymerized in tetrahydrofuran in the presence of pyridium p-toluenesulfonate as a catalyst. The polymerization proceeded by a step-growth self-polyaddition mechanism (route 2) rather than a cationic chain-reaction process (route 1). The resulting polymers possessed a polyacetal main chain instead of a carbon–carbon backbone. The copolymerization of two monomers also proceeded smoothly, generating the corresponding acetal copolymers.
Poly(ethylene terephthalate) copolymers containing nitroterephthalic units. I. Synthesis and characterization
- Pages: 3761-3770
- First Published: 30 August 2000
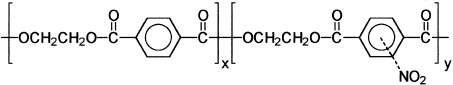
Novel poly(ethylene terephthalate) copolyesters containing nitroterephthalic units were synthesized by transesterification followed by melt copolycondensation of dimethyl terephthalate and dimethyl nitroterephthalate mixtures with ethylene glycol. Weight-average molecular weights of the polymers ranged from 10,000 to 60,000. The copolymer composition was very close to that of the feed, and we concluded that the copolyesters were statistically random. Both the melting temperature and enthalpy of the copolymers decreased with increasing amounts of nitroterephthalic units. The glass-transition temperature of the copolymers was between those of the parent homopolymers poly(ethylene terephthalate) (80 °C) and poly(ethylene nitroterephthalate) (88 °C). The copolyesters were thermally stable up to 300 °C.
Syntheses and characterizations of thermally reworkable epoxy resins II
- Pages: 3771-3782
- First Published: 31 August 2000
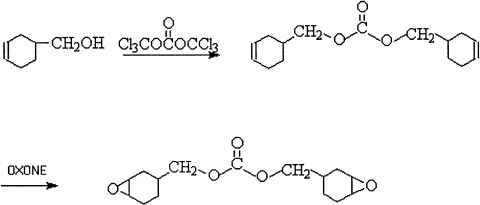
To meet the need of reworkable epoxy resins for flip chip application four cycloaliphatic epoxides containing thermally cleavable carbonate linkages have been synthesized and characterized. These materials are shown to undergo curing reactions with cyclic anhydride similarly to a commercial cycloaliphatic diepoxide. Furthermore, these cured epoxides start to decompose at temperatures lower than 350 °C, the decomposition temperature for the cured sample of the commercial cycloaliphatic diepoxide. Two formulations based on two carbonate-containing diepoxides start network breakdown around 220 °C, which is the targeted rework temperature. These two formulations are potential candidates for a successful reworkable underfill.
Dispersion polymerization of 2-hydroxyethyl methacrylate in supercritical carbon dioxide
- Pages: 3783-3790
- First Published: 30 August 2000

Poly(2-hydroxyethyl methacrylate) could be effectively emulsified in supercritical carbon dioxide using a diblock copolymer surfactant consisting of polystyrene and poly(1,1-dihydroperfluorooctyl acrylate) and the successful stabilization of the polymerization simultaneously gave spherical particles in submicron range.
Block copolymers with Zwitterionic groups at specific sites: Synthesis and aggregation behavior in dilute solutions
- Pages: 3791-3801
- First Published: 01 September 2000
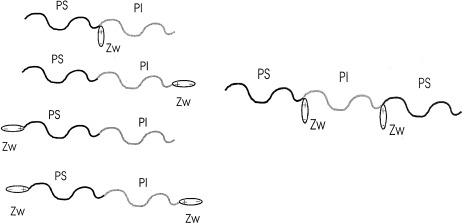
Linear block copolymers of styrene and isoprene with sulfobetaine groups at specific sites were synthesized by anionic polymerization techniques. The influence of the zwitterionic group position on the aggregating properties of these functionalized copolymers was studied in dilute solutions in the nonpolar nonselective solvent CCl4.
Kinetics, polymer molecular weights, and microstructure in zirconocene-catalyzed 1-hexene polymerization
- Pages: 3802-3811
- First Published: 01 September 2000
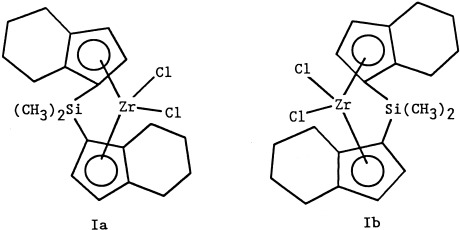
1-Hexene was polymerized by rac-(dimethylsilyl)bis(4,5,6,7-tetrahydro-1-indenyl)zirconium dichloride (I) catalyst and methylaluminoxane cocatalyst over the temperature range 0–100 °C. The polymerization rate, polymer molecular weight, and polymer microstructure (stereospecificity and regiospecificity) were studied as a function of the temperature and the concentrations of monomer, catalyst, and cocatalyst.
Polytetrahydrofuran amphiphilic networks. I. Synthesis and characterization of polytetrahydrofuran acrylate ditelechelic and polyacrylamide-l-polytetrahydrofuran networks
- Pages: 3812-3820
- First Published: 01 September 2000
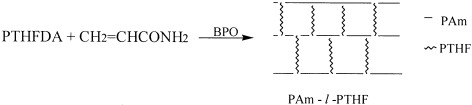
A series of novel amphiphilic polyacrylamide-l-polytetrahydrofuran (PAm-l-PTHF) networks were prepared by the free-radical copolymerization of hydrophobic ditelechelic polytetrahydrofuran acrylate (PTHFDA) with hydrophilic acrylamide. The networks can swell both in organic solvents and in water. The swelling of the networks in different solvents is composition-dependent.
RAPID COMMUNICATION
Atom transfer radical polymerization of 2-(dimethylamino)ethyl methacrylate in aqueous media
- Pages: 3821-3827
- First Published: 01 September 2000
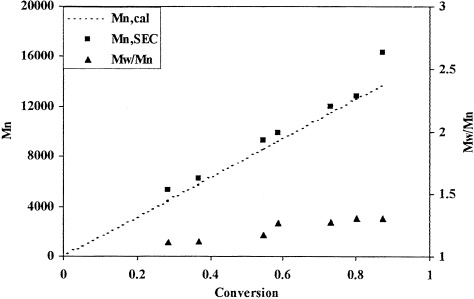
2-(Dimethylamino)ethyl methacrylate (DMAEMA) was polymerized by aqueous atom transfer radical polymerization (ATRP) with CuBr/2,2′-bipyridine (Bpy) as a catalyst and methyl α-bromophenylacetate (MBP) as an initiator at room temperature. The resulting poly(DMAEMA) gave a linear molecular weight/conversion relationship and a narrow molecular weight distribution (weight-average molecular weight/number-average molecular weight ∼ 1.2). Six initiators and two ligands were screened, and MBP/Bpy was the best for the ATRP of DMAEMA in water.
Articles
Pendent polymers of 1-methyl-2-oxo-4,5-dicyanoimidazole and their electrochemical properties
- Pages: 3828-3838
- First Published: 07 September 2000
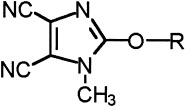
The electron-accepting 1-methyl-4,5-dicyanoimidazole group was attached to vinyl polymers, via an alkoxy link, by nucleophilic aromatic substitution (NAS) of 1-methyl-2-fluoro-4,5-dicyanoimidazole (1). The cyclic voltammetry (CV) studies show that monomeric and oligomeric model compounds are electrochemically quasi-reversible, and the degree of reversibility decreases as dicyanoimidazoles become more proximate within a molecule.
CuI and CuII salts of group VIA elements as catalysts for living radical polymerization initiated with sulfonyl chlorides
- Pages: 3839-3843
- First Published: 05 September 2000
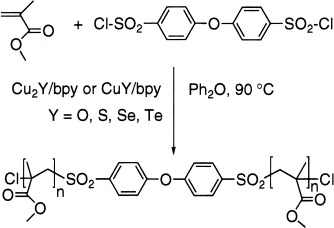
A new class of nonacidic Cu2Y/Bpy and CuY/Bpy catalysts (where Y is O, S, Se, or Te and Bpy is 2,2′-bipyridine) for the living radical polymerization of vinylic monomers initiated from sulfonyl halides is described. A polymerization mechanism based on the in situ generation of the CuCl/CuCl2 pair is suggested. The experimental rate constants of polymerization were higher for the Cu2Y-based catalysts and increased for both the Cu2Y and CuY catalysts in the order Y = O < S < Se < Te.