Long-term adherence with non-invasive ventilation improves prognosis in obese COPD patients
Abstract
Background and objective
Long-term non-invasive ventilation (NIV) has become a widespread modality of treatment in chronic obstructive pulmonary disease (COPD) patients with chronic respiratory failure. However, benefits in terms of patient-related outcomes are still under debate. Both NIV adherence and heterogeneous responses in different COPD phenotypes may contribute to the difficulty of demonstrating NIV benefits. Our aim was to assess the impact of NIV adherence on the rate of hospitalization for acute exacerbation and death.
Methods
This is a prospective multi-centre cohort study of COPD patients treated by long-term NIV. Comorbidities, anthropometrics, respiratory parameters were collected at inclusion in the study. Follow-up data included vital status, NIV adherence and hospitalizations. The influence of NIV adherence on prognosis was tested using an adjusted Cox model. Sensitivity analyses for obese and non-obese COPD subtypes were also conducted.
Results
Two hundred thirteen patients (48% obese) were included with 45.5% died during 47.7 [interquartile range = 27.8; 73] months' follow-up. Survival was better in obese COPD than non-obese COPD. The use of NIV > 9 h/day was associated with an increased risk of death or hospitalization for acute exacerbation [HR = 1.6; 95CI: 1.1–2.4]. In obese COPD, this risk described a U-shaped curve from >1 to >9 h/day NIV usage with an improvement in prognosis when NIV adherence was > 5 h/day [HR = 0.5; 95CI: 0.2–0.9].
Conclusions
Adherence to NIV was associated with better prognosis only in obese COPD. NIV use > 9 h/day predicted poor outcomes.
Abbreviations
-
- BMI
-
- body mass index
-
- COPD
-
- chronic obstructive pulmonary disease
-
- EPAP
-
- expiratory positive airway pressure
-
- FEV1
-
- forced expiratory volume in 1 s
-
- FVC
-
- forced vital capacity
-
- IPAP
-
- inspiratory positive airway pressure
-
- IQR
-
- interquartile range
-
- IRB
-
- institutional review board
-
- LT-NIV
-
- long-term non-invasive ventilation
Introduction
Since the early 1990s, long-term non-invasive ventilation (LT-NIV) has become a widespread modality of treatment for chronic obstructive pulmonary disease (COPD) patients at the stage of chronic respiratory failure.1-3 Although a small number of studies have suggested that NIV may slightly improve survival4 or may reduce hospital admissions,5, 6 the benefits of NIV in the long term for COPD patients remain uncertain compared with the clear demonstration of its effectiveness for severe COPD exacerbations, and it cannot be excluded that there is no benefit.7 Not only is its effectiveness in question but also its feasibility, as COPD patients exhibit the lowest probability of pursuing NIV or being adherent to NIV on a long-term basis.8, 9
There are several factors that may contribute to the difficulty in providing evidence of NIV benefits in COPD patients with chronic respiratory failure. First, COPD is a heterogeneous condition with different phenotypes that are associated with different probabilities for health-related events and different mortality rates.10-13 These different phenotypes may also respond differently to therapeutic strategies, in particular NIV. In a recent study, Carrillo et al.14 have shown that one-third of consecutive COPD patients admitted to the intensive care unit for acute respiratory failure requiring NIV were obese. This subgroup of patients is close to the recently described ‘systemic COPD’ phenotype exhibiting less severe airflow obstruction but a higher proportion of obesity, more cardiovascular and metabolic comorbidities than the ‘respiratory COPD’ phenotype which is characterized by severe airflow limitation without obesity.10, 11, 13 Although the relationship between respiratory function and nutritional status remains incompletely understood,15 the impact of NIV has not been addressed across these different COPD phenotypes in existing studies, and most of them4, 5, 16, 17 have excluded the overlap syndrome patients (i.e. COPD plus obstructive sleep apnoea syndrome) which are potentially the better responders to NIV.18, 19 Second, a sufficiently long daily use of NIV may be important in order to improve clinical outcomes.16, 17, 20 In McEvoy et al.'s study,4 survival advantage was greater in the per-protocol analysis (i.e. restricted to patients who used NIV treatment more than 4 h/night), suggesting that the nightly duration of NIV treatment was an important determinant of outcomes. However, in previously published studies, daily NIV adherence is often only reported as ‘a mean daily use’15 with compliant patients being defined using arbitrary thresholds of nightly usage.4
Therefore, the main objective of the study was to assess the relationship between daily NIV use and outcome (i.e. composite outcome of death or hospitalization for acute exacerbation21). To address this question properly, we aimed to analyse adherence as a time-dependent variable without any pre-existing threshold to separate compliant versus non-compliant patients. Moreover, because nutritional status, a classical and robust prognostic factor in COPD patients,22 has also been found independently associated with prognosis in those treated with LT-NIV,15 we also aimed to assess whether NIV adherence may differently influence the prognosis of COPD patients according to this nutritional status using two simple categories: obese COPD patients (BMI ≥ 30 kg/m2) and non-obese COPD patients (BMI < 30 kg/m2).
Methods
Study design and patient selection
Since January 2008, a multicentric cohort of patients with COPD treated by long-term home NIV has been implemented by six French medical facilities [four university hospitals and two rehabilitation centres]. COPD patients were included at discharge from hospital with long-term NIV, either after NIV initiation or after a regular medical visit to check NIV efficacy in patients already on treatment. The diagnosis of COPD, ascertained by one of the physician investigators, was based on the patient's clinical history and a post-bronchodilator FEV1/FVC ratio <70%. Patients with neuromuscular diseases were excluded. In France, LT-NIV is indicated for COPD when (i) diurnal PaCO2 ≥ 55 mm Hg; (ii) in cases of documented nocturnal hypoventilation; (iii) there are recurrent hospitalizations for exacerbations. Many centres also use NIV as first-line treatment for hypercapnic overlap syndromes (COPD associated with sleep apnoea). Our institutional review board (IRB-6705) approved this study and all patients included signed a written consent. The clinical trial registration number was NCT 01192451.
Data collection, follow-up and outcomes
Baseline characteristics, including medical history, anthropometric data and respiratory parameters were collected at inclusion from patients’ medical records. The condition at NIV initiation (acute respiratory failure or chronic respiratory failure) was also reported. Arterial blood gas values were considered even when they were performed under supplemental O2.
Follow-up data were collected using: (i) data from regular medical visits scheduled to check NIV efficacy and to provide settings adjustment when necessary; (ii) data from unexpected hospitalization for an acute medical condition (respiratory and non-respiratory causes); (iii) data from home care providers. In France, health-care authorities require a systematic home-based follow-up (every 3–6 months) by home care nurses or technicians. During this follow-up, adherence is objectively measured at every visit from the NIV built-in time counters. Vital status was determined either by the patient's home care provider in case of death at home or by the physician in charge of the patient in case of death during hospitalization. All causes of death were considered in the present analysis. Hospital admissions for an acute episode of COPD were documented from hospital admission records. The data were censored on 1 November 2012.
Data management and statistical analysis
Characteristics are described using frequency and percentage for qualitative variables and median with interquartile range (IQR) for quantitative ones.
Three steps of analysis were used to assess whether the duration of daily NIV use, a time-dependent variable, influenced long-term prognosis in patients with COPD. The first step was to compare characteristics of ‘obese COPD’ and ‘non-obese COPD’ and to compare patients’ survival probability accordingly with these two subtypes. Differences in characteristics between the two subtypes were tested using a chi-square test or a Mann–Whitney non-parametric test. Survival curves were plotted using Kaplan–Meier estimates and were compared between obese and non-obese COPD patients using a log–rank test. In a second step, univariate log–rank tests were used to determine baseline variables associated with the risk of death or the first hospital readmission for an acute episode of COPD. Age was sorted in four categories (≤60; [60; 70]; [70; 75]; >75 years) and BMI in three (<21; [21–30]; ≥30 kg/m2). Other continuous variables were dichotomized to the median values. After imputation for missing data using sequential regression models (from the least missing to the most missing variable) variables which were associated with prognosis (with a P-value < 0.2) were entered into stepwise selection procedure in a multivariate Cox model. Finally, the influence of NIV adherence (considered as a time-dependent variable) on prognosis was tested using a Cox model adjusted for other baseline variables associated with the prognosis. Sensitivity analyses for obese and non-obese COPD subtypes were also conducted. All statistical analysis was performed using SAS 9.3 (Cary, NC, USA).
Results
Characterization of patients and comparison between obese and non-obese COPD treated with long-term NIV
A total of 246 patients were included in the study. Thirty-three patients (13.4%) were secondarily dropped from the present analysis because data on NIV adherence were unavailable. The median [IQR] follow-up duration was 47.7 [27.8–73] months. One hundred two patients (48%) were obese. Table 1 gives the baseline characteristics for the whole group and for the two COPD subtypes. At date of censoring, 97 patients (45.5%) had died, 64 of them being non-obese. Non-obese COPD patients had lower respiratory parameters, were more often treated with long-term oxygen therapy before starting NIV, and NIV was more often started during an acute respiratory failure. In contrast, obese COPD patients exhibited higher proportions of cardiometabolic comorbidities such as diabetes, sleep apnoea syndrome and systemic hypertension. As shown in Figure 1, non-obese COPD subtype was associated with a higher rate of death or hospital readmission than obese COPD; 1-year and 3-year Kaplan–Meier estimates for death or hospital readmission were respectively of 31% and 58% for non-obese COPD versus 13% and 27% for obese COPD. In terms of NIV settings, expiratory (EPAP) and inspiratory (IPAP) positive airway pressures were set a higher levels in obese COPD; however, pressure support (IPAP minus EPAP) did not differ between the two groups. Table 2 reports follow-up data according to the COPD subtypes. Interestingly, average daily NIV use during follow-up was longer in obese COPD than in non-obese COPD.
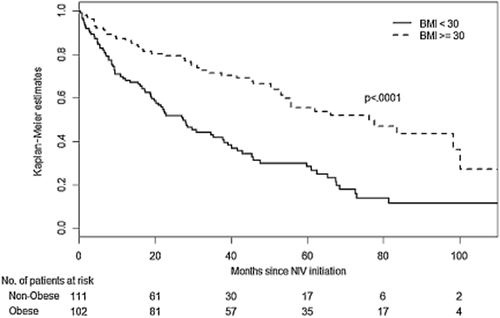
Kaplan–Meier estimates of survival without hospital readmission for acute exacerbation according to the two subtypes of chronic obstructive pulmonary disease (obese and non-obese). BMI, body mass index; NIV, non-invasive ventilation.
| Whole group | Obese COPD | Non-obese COPD | P-value | |
|---|---|---|---|---|
| n = 213 | n = 102 | n = 111 | ||
| Age, years | 68 [60–75] | 66 [58–74] | 71 [61–76] | 0.0390 |
| Gender, % male | 77 | 82 | 72 | 0.0700 |
| BMI, kg/m2 | 29.8 [23.7–34] | 34.5 [32.2–38.6] | 23.9 [20.9–27] | <0.0001 |
| Urban living (≥5000 inhabitants), % | 49 | 48.5 | 49.5 | 0.900 |
| PaO2, mm Hg | 70.5 [65–77.3] | 70.1 [65.5–76.1] | 71.1 [64.5–79.1] | 0.600 |
| PaCO2, mm Hg | 48.5 [43.9–52.9] | 47.0 [41.6–51.3] | 49.8 [46.4–53.9] | 0.0080 |
| [HCO3] mEq/L | 29.8 [27.5–31.9] | 28.8 [26.2–30.5] | 30.6 [28.7–32.5] | <0.0001 |
| FVC, mL | 2075 [1680–2670] | 2275 [1735–3035] | 1903 [1538–2340] | 0.0006 |
| FVC, % of predicted value | 64.2 [51–74] | 66.6 [55.6–78.3] | 61.6 [48.3–72.6] | 0.0090 |
| FEV1, % of predicted value | 42.5 [31–56] | 50.3 [39.8–63.3] | 36.0 [26.7–45] | <0.0001 |
| FEV1/FVC, % | 0.55 [0.45–0.62] | 0.62 [0.56–0.65] | 0.48 [0.39–0.54] | <0.0001 |
| Medical history | ||||
| Hospitalization, 2 preceding years, % | 74.4 | 76.8 | 72.2 | 0.5000 |
| NIV started in acute condition, % | 53.5 | 39.2 | 66.7 | <0.0001 |
| History of smoking, % | 86.2 | 83.5 | 88.8 | 0.3000 |
| Hypertension, % | 50.2 | 63.7 | 37.8 | 0.0002 |
| Heart failure,% | 23.5 | 27.5 | 19.8 | 0.1900 |
| Diabetes, % | 26.8 | 39.2 | 15.3 | <0.0001 |
| Sleep apnoea, % | 45.5 | 72.5 | 20.7 | <0.0001 |
| Previous long-term O2 therapy, % | 29.7 | 19.6 | 39.1 | 0.0020 |
| NIV settings | ||||
| IPAP (cmH2O) | 19 [16.5–21] | 20 [18–22] | 18 [16–20] | 0.0002 |
| EPAP (cmH2O) | 7.6 [6–10] | 8 [6–10] | 6.1 [5–8] | <0.0001 |
| Pressure support (cmH2O) | 11 [10–12] | 11 [9–12] | 11 [10–13] | 0.7000 |
| Back-up rate (per minute) | 15 [12.4–16] | 14 [12–16] | 15 [13–16] | 0.1800 |
- The last column shows P-values for comparisons of characteristics between obese and non-obese COPD patients (chi-square test or a Mann–Whitney non parametric test).
- Pressure support = IPAP − EPAP.
- BMI, body mass index; COPD, chronic obstructive pulmonary disease; EPAP, expiratory positive airway pressure; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IPAP, inspiratory positive airway pressure; PaO2, partial arterial oxygen concentration; PaCO2, partial arterial carbon dioxide concentration.
| Whole group | Obese COPD | Non-obese COPD | P-value | |
|---|---|---|---|---|
| n = 213 | n = 102 | n = 111 | ||
| Mean daily use of NIV, h/day | 5.9 [3.9–8.3] | 6.9 [4.1–8.6] | 5.3 [3.2–7.9] | 0.0070 |
| Follow-up duration, months | 47.7 [27.8–73] | 55.9 [33.1–77.7] | 38.5 [22.6–69.7] | 0.0020 |
| Rate of death, % | 45.5 | 32.4 | 57.7 | 0.0002 |
- The last column shows P-values for comparisons of follow-up data between obese and non-obese COPD patients (chi-square test or a Mann–Whitney non parametric test).
- COPD, chronic obstructive pulmonary disease; NIV, non-invasive ventilation.
Baseline variables associated with the risk of death or hospital readmission for acute exacerbation of COPD
Table 3 shows baseline variables associated with prognosis in univariate analysis. In multivariable analysis (Table 4), older age, lower BMI, sleep apnoea syndrome, previous long-term oxygen therapy before starting NIV, the condition of NIV initiation (acute versus chronic condition) and lower FEV1/FVC ratio remained independently associated with the poor prognosis.
| % of patients alive without rehospitalization (n = 86) | % of patients dead or readmitted to hospital (n = 127) | P-value | ||
|---|---|---|---|---|
| Age (years) | ≤60 | 54.1 | 45.9 | 0.0200 |
| [60; 70] | 43.9 | 56.1 | ||
| [70; 75] | 35.7 | 64.3 | ||
| >75 | 24.5 | 75.5 | ||
| Gender | Female | 40.8 | 59.2 | 0.3000 |
| Male | 42.1 | 57.9 | ||
| Urban living (≥5000 inhabitants) | No | 40.2 | 59.8 | 0.4000 |
| Yes | 40.6 | 49.4 | ||
| BMI kg/m2 | <21 | 14.3 | 85.7 | <0.0001 |
| [21–30] | 32.5 | 67.5 | ||
| ≥30 | 53.9 | 46.1 | ||
| PaO2, mm Hg | ≤70 | 39.4 | 60.6 | 0.9000 |
| >70 | 41.2 | 58.8 | ||
| PaCO2, mm Hg | ≤49 | 45.7 | 54.3 | 0.0020 |
| >49 | 34.0 | 66.0 | ||
| [HCO3-], mEq/L | ≤30 | 46.1 | 53.9 | 0.0040 |
| >30 | 33.7 | 66.3 | ||
| FEV1, % of predicted value | >42.5 | 53.2 | 46.8 | 0.0040 |
| ≤42.5 | 33.1 | 66.9 | ||
| FEV1/FVC, % | ≤0.6 | 31.0 | 69.0 | <0.0001 |
| >0.6 | 60.3 | 39.7 | ||
| Medical history | ||||
| Hospitalized (2 preceding years) | No | 40.4 | 59.6 | 0.8000 |
| Yes | 40.4 | 59.6 | ||
| NIV started in acute condition | No | 51.5 | 48.5 | <0.0001 |
| Yes | 30.7 | 69.3 | ||
| History of smoking | No | 51.2 | 48.8 | 0.0700 |
| Yes | 37.8 | 62.2 | ||
| Hypertension | No | 40.6 | 59.4 | 0.8000 |
| Yes | 40.2 | 59.8 | ||
| Heart failure | No | 43.6 | 56.4 | 0.1400 |
| Yes | 30.0 | 70.0 | ||
| Diabetes | No | 39.1 | 60.9 | 0.2100 |
| Yes | 43.9 | 56.1 | ||
| Sleep apnoea syndrome | No | 38.8 | 61.2 | 0.0300 |
| Yes | 42.3 | 57.7 | ||
| Previous oxygen therapy | No | 46.7 | 53.3 | <0.0001 |
| Yes | 25.4 | 74.6 | ||
| NIV settings (at hospital discharge) | ||||
| IPAP (cmH2O) | ≤19 | 43.6 | 56.4 | 0.2500 |
| >19 | 36.5 | 63.5 | ||
| EPAP (cmH2O) | ≤8 | 35.7 | 64.3 | 0.03700 |
| >8 | 49.3 | 50.7 | ||
| Pressure support (cmH2O) | ≤11 | 48.5 | 51.5 | 0.0900 |
| >11 | 33.0 | 67.0 | ||
| Back-up rate (per minute) | ≤14 | 47.0 | 53.0 | 0.0560 |
| >14 | 34.5 | 65.5 |
- The last column shows P-values of log–rank tests used to determine baseline variables associated with the risk of death or the first hospital readmission for an acute episode of COPD. Age was sorted in four categories (≤60; [60; 70]; [70; 75]; >75 years) and BMI in three (<21; [21–30]; ≥30 kg/m2). Other continuous variables were dichotomized to the median values.
- BMI, body mass index; COPD, chronic obstructive pulmonary disease; EPAP, expiratory positive airway pressure; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IPAP, inspiratory positive airway pressure; PaO2, partial arterial oxygen concentration; PaCO2, partial arterial carbon dioxide concentration.
| Parameter estimate | Standard error | Hazard ratio | 95% Hazard ratio confidence limits | P-value | |||
|---|---|---|---|---|---|---|---|
| Age, years | ≤ 60 | 0.000 | 1.00 | 0.0100 | |||
| [60; 70] | −0.009 | 0.26 | 0.99 | 0.59 | 1.66 | ||
| [70; 75] | 0.530 | 0.28 | 1.70 | 0.98 | 2.96 | ||
| >75 | 0.670 | 0.26 | 1.96 | 1.19 | 3.23 | ||
| BMI, kg/m2 | <21 | 0.430 | 0.26 | 1.54 | 0.94 | 2.55 | 0.0200 |
| [21–30] | 0.000 | 1.00 | |||||
| ≥30 | −0.420 | 0.24 | 0.66 | 0.41 | 1.05 | ||
| Sleep apnoea syndrome | 0.500 | 0.22 | 1.65 | 1.08 | 2.51 | 0.0200 | |
| Previous oxygen therapy | 0.390 | 0.20 | 1.48 | 1.00 | 2.20 | 0.0500 | |
| NIV started in acute condition | 0.520 | 0.20 | 1.68 | 1.14 | 2.48 | 0.0090 | |
| FEV1/FVC | >0.6 | −1.020 | 0.27 | 0.36 | 0.22 | 0.61 | 0.0001 |
- Age was sorted in four categories (≤ 60; [60; 70]; [70; 75]; >75 years) and BMI in three (<21; [21–30]; ≥30 kg/m2). FEV1/FVC was dichotomized to the median value.
- The last column shows P-values of multivariate Cox model (stepwise selection procedure).
- BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; NIV, non-invasive ventilation.
Influence of daily duration of NIV use on prognosis of patients with COPD
Figure 2 displays the adjusted hazard ratios (HR) for the risk of death or hospital readmission according to the daily length of NIV use in the whole group and in the two subgroups of COPD patients. In obese COPD (Fig. 2b), this risk described a U-shaped curve from >1 to >9 h/day with a significant improvement in prognosis when use of NIV was >5 h/day [HR = 0.5; 95% CI: 0.2–0.9]. For the whole group (Fig. 2a), use for above 9 h/day was associated with a higher risk of death or hospital readmission.
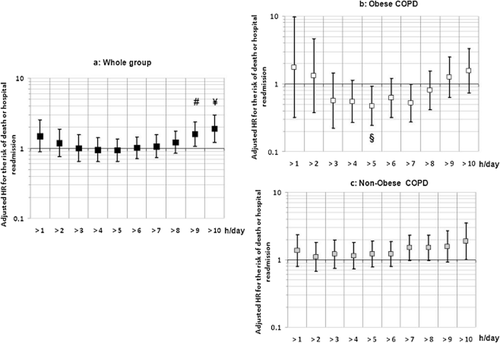
Adjusted hazard ratio (HR) for the risk of death or hospital readmission for acute exacerbation of chronic obstructive pulmonary disease (COPD) according to the daily use of non-invasive ventilation (NIV). (a) whole group; (b) obese COPD; (c) non-obese COPD. The figure displays the association of NIV use (hazard ratio) with the risk of death or hospital readmission for acute exacerbation of COPD when moving the cut-off of NIV use from 1 to 10 h a day (Cox model adjusted for age, body mass index (BMI), Sleep apnoea syndrome, previous oxygen therapy, condition of NIV initiation, ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC). Symbols show significant hazard ratio (#: P = 0.022; ¥: P = 0.005; §: P = 0.029).
Discussion
The main results can be summarized as follows: (i) despite NIV use, more than 40% of the patients died during follow-up (median 47.7 months). Older age, lower BMI, sleep apnoea syndrome, previous long-term oxygen therapy before starting NIV, the condition at NIV initiation and lower respiratory function were all variables independently associated with prognosis. (ii) ‘Non-obese COPD exhibited poorer prognosis and also lower mean daily NIV usage than ‘obese-COPD’ patients. (iii) Use of NIV above 9 h/day, reflecting a potential marker of severity of the disease, was associated with an increased risk of death or hospitalization for acute exacerbation. In obese COPD, this risk described a U-shaped curve from >1 to >9 h/day with a significant improvement in prognosis when NIV was used >5 h/day.
In spite of NIV treatment, in the present study, the overall mortality among COPD patients remained dramatically high, similar to that in a previously published study with a comparable median follow-up.18 We demonstrated that the prognosis under NIV treatment is highly dependent on the COPD subtype; non-obese COPD showing a limited response to NIV compared with obese COPD. Although, in the present study, the repartition of patients was limited to the body mass index, our results clearly show that obese patients also exhibited a better respiratory function but higher proportions of cardiovascular and metabolic risk factors than non-obese patients. The concept of different COPD subtypes is not completely new;23 however, recent studies10, 11 have convincingly established that not only airflow obstruction but also extra-pulmonary comorbidities have an important impact on the prognosis of COPD patients. In line with our results, these previous studies have demonstrated that patients characterized by low respiratory function and lower body mass index, so-called ‘respiratory COPD’, had higher rates of death than ‘systemic COPD’. This ‘systemic COPD’ phenotype, in accordance with our results, is characterized by mild airway obstruction but higher rates of cardiovascular disorders, diabetes and obesity. The fact that sleep apnoea was an independent negative prognostic factor while obesity was a positive one seems counter-intuitive since obesity is one of the most important risk factor for the development of obstructive sleep apnoea. Obesity is a robust positive prognostic factor in COPD as in others chronic conditions so-called ‘obesity paradox’; yet, this factor, only defined by body mass index, gathers a large heterogeneity in clinical features and sleep apnoea would presumably be a marker of advanced cardiometabolic comorbidities. The previous randomized controlled trials addressing NIV efficacy in COPD have mostly excluded patients with significant cardiovascular4, 5, 16 or metabolic comorbidities,16 or concurrent sleep apnoea syndrome (overlap syndrome).4, 5, 16, 17, 20 Yet, overlap syndrome is the one COPD subgroup about whom there is general agreement about the merits of positive pressure treatment. These studies showing a limited impact of NIV actually focused on the ‘respiratory non-obese COPD’ phenotype. Although this was justified for preventing inclusion bias in randomized control trials, it could have led to underestimate NIV efficacy in obese systemic COPD. Indeed, systemic comorbidities are common in COPD and the so-called ‘COPD comorbidome’ strongly influences survival.24 Thus excluding these COPD phenotypes from interventional studies does not reflect the reality of current clinical practice.14, 24 It clearly limits recruitment rates in the trials (see flow chart of the McEvoy et al. trial4) and prevents the eventual impact of NIV on extra-pulmonary markers to be observed, which may contribute to demonstrate its efficacy. Moreover, from a respiratory physiology point of view, obese patients are more likely to hypoventilate especially during sleep owing to obesity-related abnormalities in respiratory mechanics. NIV has the capability to recruit peripheral airways and increase alveolar ventilation in a well-preserved lung, reduce respiratory muscles work and suppress REM sleep hypoventilation. In contrast, NIV could be less effective for addressing V/Q mismatch in non-obese COPD patients with more generalized emphysema.25 Thus our data in an observational cohort provide a strong rationale to design new randomized controlled trials targeting NIV effectiveness in the systemic COPD phenotypes.
Another open question in non-invasive ventilation in COPD patients is poor treatment adherence. The current study underscores a potential interaction between COPD phenotypes and daily NIV usage. Non-obese COPD patients who clearly demonstrated a lower survival benefit from NIV also exhibited a lower average daily NIV adherence. There are probably different causes underlying this relatively low adherence to treatment. An imbalance between constraints associated with NIV treatment and self-perceived improvement could lead to non-adherence. Also, in these non-obese COPD patients, with low respiratory function and possible hyperinflation, ventilator settings are more difficult to adjust and may impact subjective sleep quality.26 One limitation of our study is that we did not have systematic sleep studies under NIV.
Contrasting with the situation of non-obese COPD, our results showed that NIV adherence positively influenced the rate of hospitalization and death with a dose effect relationship in obese COPD. By analogy, for sleep apnoea treatment with CPAP, a dose effect response is now systematically reported to improve not only symptoms27, 28 but also cardiometabolic outcomes.29, 30 The way we used to analyse NIV adherence (i.e. as a time-dependent variable without a pre-existing threshold) was original and convincingly graphically demonstrates the impact of NIV adherence on prognosis. Again, this relationship was true only in obese COPD patients. The range of NIV response in non-obese COPD is probably too small to demonstrate any effect of adherence to treatment. Moreover, prolonged daily use, which may possibly reflect severity of the disease, was associated with poor outcomes. Such a degree of NIV dependence should be considered as harmful by caregivers. As demonstrated by Funk et al., in such patients any reduction in NIV use rapidly led toward clinical worsening and ICU admission for acute respiratory failure.20 The evolution of NIV use should be investigated in future studies, addressing the interest of telemedicine programs in COPD patients on home-based NIV.
Finally, although the method used to analyse NIV adherence provides a new perspective on the role of NIV in patients with COPD, we acknowledge that the initial phenotyping of the patients was limited as many patients were initiated on NIV in acute conditions; particularly, no detail was collected regarding apnoea–hypopnoea index, systolic/diastolic blood pressure, glucose blood level. We only collected the presence (or not) of each main comorbidity. Moreover, several important clinical or biological features (such as dyspnoea scales, exercise capacity, low-grade inflammation, anemia, etc.), which are known to contribute to the prognosis, were not included in the present analysis. Thus, it is impossible to rule out their possible impact on the present results.
In conclusion, our study is the first demonstrating that adherence to LT-NIV, analysed as a time-dependent variable and without a pre-existing threshold, positively influences the prognosis of obese COPD patients who are close to ‘systemic COPD’. Although the best phenotype of COPD patient who would benefit from LT-NIV needs to be better defined, a future randomized controlled trial should be dedicated primarily to ‘systemic COPD’. In non-obese COPD, it cannot be excluded that there is no benefit of LT-NIV. Finally, our study is important as proposing two important clinical messages: First, 5 h/day of NIV adherence could be a goal to reach in obese COPD patients for significant impact on prognosis. Second, NIV adherence above 9 h/day may be considered as a marker of severity and such adherence should alert clinicians to follow these patients carefully
Acknowledgements
We thank Dr. Alison Foote for English editing and Stephane Ruckly for the revision of the statistical analysis. The RIO-VNI was sponsored by Grenoble University Hospital. The project was funded by a ‘Programme Hospitalier de recherche Clinique’ (n°API 26-07). Unrestricted research grants were also obtained from Agir à Dom (Grenoble, France) and ALLP (Lyon, France).




