The Molecular Legacy of Apoptosis in Transplantation
Abstract
Transplanted organs have to cope with diverse immunologic and metabolic stressors that augment the percentage of stressed and dying cells. Cell death, whether apoptotic or necrotic, is crucial in various transplantation-associated conditions. Necrosis, a proinflammatory type of cell death classically considered as accidental, is increasingly recognized as a highly controlled death program. Apoptosis, the classical programmed cell death mode program, is tightly orchestrated and culminates in the activation of caspases. Apoptosis was classically regarded as a silent form of cell death, but mounting evidence indicates that apoptotic cells “don't go silently” and leave a heritage to the local microenvironment. This apoptotic legacy, embedded within the effector phase of apoptosis, is aimed, at least in part, at controlling leukocyte trafficking and fostering tissue remodeling at sites of apoptotic cell deletion and can promote maladaptive remodeling pathways of importance for obliterative vascular remodeling. Moreover, apoptotic cells can transfer bioactive molecules by the release of apoptotic membrane vesicles that, in turn, shapes the phenotype and functions of immune cells. In this review, we summarize recent data highlighting the importance of apoptosis-associated intercellular communication networks in the regulation of allograft remodeling and immune responses in transplantation.
Introduction
Transplanted organs are exposed to various types of cellular stresses (ischemic, immunologic, toxic, etc.) that compromise tissue viability and promote inflammation. Ischemia-reperfusion injury and allograft rejection are among the most important tissue stressors in transplant medicine. These conditions result in increased intragraft cell death and may foster maladaptive healing and scarring. Increased cell death may also shape innate and adaptive immune responses, regulating the balance between tolerance and rejection of allogenic tissue.
Mounting evidence suggests that cell death programs and tissue remodeling are not independent processes and that various levels of interaction are weaved into these pathways. Stressed cells undergoing programmed death (of either apoptotic or necrotic form) can communicate with their microenvironment and engage in intercellular crosstalk of central importance in inflammation and tissue remodeling. Indeed, the molecular machinery controlling the death program favors the extracellular translocation of a highly regulated set of mediators representing the “legacy” of dying cells to the local microenvironment. This molecular legacy mounts a communication network with surrounding cells, preparing injured tissues for remodeling and repair.
Cell death can also foster intercellular communication through the release of membrane vesicles able to coordinate important stages of the immune response, such as leukocyte recruitment and antigen presentation. Apoptotic cells can transfer bioactive molecules by the release of apoptotic bodies that, in turn, shapes the phenotype and functions of recipient cells. These nonconventional crosstalk pathways, mediated by membrane vesicles released during cell death, are crucial in modulating alloimmune responses in transplantation settings.
Here, we provide a comprehensive overview of the mechanisms and consequences of the programmed cell death legacy and unconventional intercellular trafficking in transplantation. We review the paracrine outcomes of apoptosis in tissue remodeling and transplant vasculopathy (TV), a major cause of graft loss in kidney transplant. We also describe the implications of apoptotic membrane vesicles in the modulation of alloimmunity and discuss the potential clinical applications of monitoring the endothelial apoptotic legacy.
Apoptosis Is Not a Silent Way to Die
Apoptosis is a programmed cell death pathway characterized by rounding-up of cells, plasma membrane blebbing, cell volume reduction, chromatin condensation and nuclear fragmentation, leading to the generation of apoptotic bodies (1–3). Caspases, a family of aspartate-specific proteases, are master molecular regulators of the apoptotic program. Upon receiving a proapoptotic stimulus (either mitochondrial-dependent or death domain-dependent), initiator caspases 2, 8, 9 and 10 trigger downstream executioner caspases 3, 6 and 7 by proteolytic processing. Importantly, caspase activation is not synonymous with apoptosis because caspases 1, 4 and 5 are involved in the regulation of inflammation through proteolytic activation of the proinflammatory cytokines IL-1β and IL-18 (4). Moreover, regulatory apoptotic caspases are implicated in nonlethal biological processes, including DNA repair, cell differentiation, erythropoiesis, immune regulation and cell cycle control.
The immunological consequences of apoptosis vary, depending on the inflammatory milieu produced by dying cells. During the early phase of apoptosis, dying cells are phagocytosed by macrophages and dendritic cells (DCs) in an antiinflammatory and tolerogenic manner. Indeed, apoptotic cells can inhibit the secretion of proinflammatory cytokines and promote the production of antiinflammatory cytokines, including TGF-β and IL-10. The cell membrane becomes permeabilized during the late phases of apoptosis, resulting in the liberation of endogenous molecules known as danger-associated molecular patterns (DAMPs) that can directly stimulate immunogenic responses by promoting cytotoxic T-cell and DC activation. DAMPs, such as uric acid or high mobility group protein 1, may activate DCs and macrophages that are equipped with pattern recognition receptors, fueling inflammatory responses. Late apoptosis and necrosis share common immunological features, as both cell death routines are associated with membrane permeabilization and the release of danger signals, triggering acute inflammatory and immunogenic reactions. Necrosis has long been considered as an accidental and nonprogrammed process until death domain receptors, including tumor necrosis factor receptor 1, Fas/CD95 and tumor necrosis factor-related apoptosis-inducing ligand Receptor, emerged as activators of necrosis, particularly in the presence of caspase inhibitors. Receptor-interacting kinase-1 and Kinase-3 evoke death domain-mediated cell death (5). Consequently, the classical dichotomy between “silent” apoptosis and “noisy” and uncontrolled necrosis needs to be revisited. A detailed description of the molecular pathways regulating programmed necrosis is beyond the scope of this article and readers are referred to recent reviews (6,7).
Paracrine Responses Associated with Apoptosis Promote Tissue Remodeling
A growing body of evidence suggests that the early stages of apoptosis, characterized by the absence of membrane permeabilization, are prone to triggering various modes of intercellular communication of importance in local homeostasis and tissue remodeling. These paracrine pathways, induced downstream of caspase-3, regulate leukocyte trafficking and activation, extracellular membrane degradation and fibrosis.
The apoptotic legacy facilitates leukocyte trafficking
Swift clearance of apoptotic debris prevents the activation of immune responses to autoantigens expressed by apoptotic bodies and favors tissue repair (8). Professional phagocytes of myeloid lineage, such as macrophages, DCs and, to a lesser extent, neutrophils, carry phagocytic receptors, facilitating the uptake of apoptotic cells and apoptotic bodies. Nonimmune cells, such as epithelial cells, may also contribute to the phagocytosis of apoptotic cells. Expression of the phosphatidylserine receptor kidney injury molecule 1 in renal tubular epithelial cells after injury confers a phagocytic phenotype, culminating in the internalization and clearance of apoptotic cells (9). This mechanism is thought to prevent intratubular obstruction and preserve tubular flow after renal ischemic injury.
Viable cells send “don't eat-me” signals to prevent their uptake by phagocytes. These include membrane receptors, such as CD46, CD47 and CD31. During apoptosis, biochemical changes elicit the release of “come-to-me” signals, exposure to “eat-me” signals and the loss of “don't eat-me” signals that collectively facilitate apoptotic cell clearance. “Come-to-me” factors, such as fractalkine and the lipid mediator lysophosphatidylcholine, both released by apoptotic cells, promote the recruitment of macrophages and monocytes (8,10,11). Loss of plasma membrane phospholipid asymmetry during apoptosis fosters exposure to anionic phospholipids, such as phosphatidylserine, which can be recognized by a number of phagocytic receptors and opsonins, including T-cell immunoglobulin mucins or milk fat globular epidermal growth factor (EGF) 8 protein, acting as “eat-me” signals for phagocytes (12).
Increased endothelial apoptosis is a major predictor of TV (13,14) and correlates with the accumulation of T cells and macrophages in chronically rejected vessels (15). “Come-to-me” and “eat-me” factors are potentially important in the recruitment of leukocytes in TV. Apoptotic endothelial cells (EC) release monocyte chemoattractant peptide-1 (MCP-1), which recruits monocytes at sites of injury. In a swine heart transplantation model, inhibition of its expression significantly reduced the number of mononuclear cells accumulating in the lumen of coronary arteries (16). Intercellular adhesion molecule-1 (ICAM-1) and VCAM-1 were overexpressed in chronically rejected vessels and apoptosis-induced EC hyperadhesiveness was shown to occur, at least in part, through ICAM-1 and VCAM-1 overexpression and β1-integrin interactions (17). To date, most paracrine mediators released or expressed on apoptotic cells have been linked with activation of effector caspase-3, indicating that the paracrine program is embedded within the effector phase of apoptosis.
The apoptotic legacy aims at fostering remodeling and repair at sites of apoptotic cells
The paracrine apoptotic program does not solely regulate leukocyte trafficking but also aims at favoring remodeling and tissue repair after cell deletion. Although potentially beneficial initially, sustained activation of repair pathways may promote fibrogenesis or maladaptive vascular remodeling. Various animal models and biopsy specimens from human renal and heart transplant recipients have demonstrated a close correlation between the presence and persistence of microvascular apoptosis and the development of maladaptive allograft vascular remodeling (13–15,18; Figure 1).
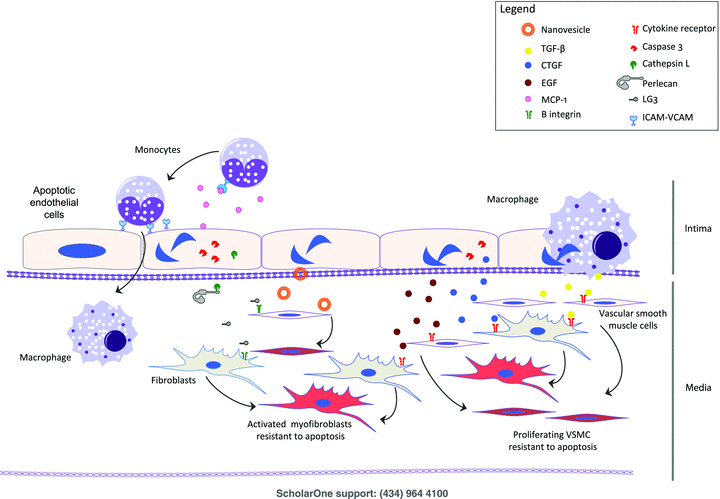
The endothelial paracrine apoptotic response in transplantation.
Production of fibrogenic factors: Caspase-3 activation on apoptotic ECs triggers the export of connective tissue growth factor (CTGF), which, in turn, functions as a fibrogenic cofactor in vitro and in vivo (19). CTGF is a central effector of allograft fibrosis in chronic heart and kidney rejection models and human renal transplant patients (20). Apoptotic EC also generates EGF (21), another factor implicated in allograft fibrosis.
Matrix proteolysis and production of cryptic, functionally active fragments: Another important aspect of the paracrine apoptotic program is the release of proteolytic enzymes that occurs in a highly regulated fashion in the absence of nonspecific cell leakage. Cathepsin L, tissue plasminogen activator and a disintegrin and metalloproteinases (ADAMs) are exported extracellularly during apoptosis via caspase-dependent pathways (22). Caspase-3 activation is central to cathepsin L externalization, which, in turn, cleaves the extracellular matrix (ECM) component perlecan, producing a truncated C-terminal fragment harboring a laminin G motif (referred to as LG3). In turn, through interactions with β1-integrins, LG3 activates extracellular signal—regulated kinase 1/2-dependent prosurvival and promigratory pathways in vascular smooth muscle cells and mesenchymal stem cells fostering neointima formation in a murine TV model (21,23). Serum and urinary LG3 levels are elevated in renal transplant patients with either acute or chronic allograft rejection (23).
Macrophage phenotype changes in response to apoptosis and their consequences in tissue remodeling: Macrophages are important mediators and predictors of acute and chronic allograft dysfunction (24). The local microenvironment plays a prominent role in the dynamic development of macrophage phenotype, including proinflammatory, antimicrobial (M1) macrophages and alternative/regulatory (M2) macrophages. The former phenotype is associated with inflammatory, cytotoxic responses, whereas the latter is linked to wound healing and fibrosis. Phagocytosis of apoptotic cells, as seen in failing allografts, promotes the acquisition of regulatory macrophage phenotypes similar to M2 macrophages (25,26). M1 macrophage activation has been coupled with decreased allograft survival (27), whereas increased, alternative activation toward the M2 phenotype has been associated with a reduction of acute and chronic injury scores in a CCR5 renal transplant knockout mouse model (28). Similar observations have been made in human allograft biopsies. Identification of alternative macrophage-activation transcripts has been correlated with better allograft survival (24,29). This suggests that an alternative/regulatory macrophage phenotype could attenuate tissue injury, favoring prolonged allograft survival. However, prolonged M2 reprogramming would promote the generation of a fibrogenic microenvironment.
The release of membrane vesicles by apoptotic cells regulates immune responses
The secretion of membrane vesicles by apoptotic cells facilitates the exchange of cellular material, including membrane and cytoplasmic proteins, messenger RNA and micro-RNA, between cells. Numerous types of secreted membrane vesicles have been described and their characterization relies on their mode of production, size, biochemical properties and isolation method (30).
Apoptosis is distinguished by the generation of apoptotic bodies, a heterogeneous class of membrane vesicles ranging in size from 50 nm to 500 nm and sedimenting at 50 000 g to 100 000 g. Apoptotic bodies originate from apoptotic cells and may carry nuclear components, such as histones (1,2). Apoptotic bodies regulate alloimmunity and may constitute novel therapeutic targets in transplantation. Systemic administration of apoptotic bodies carrying the entire repertoire of donor MHC molecules has proven effective in preventing acute rejection and prolonging graft survival in animal models of cardiac or skin transplantation (31,32; Figure 2). These experiments are based on the concept that apoptotic bodies’ internalization by resting DCs in secondary lymphoid organs plays a critical role in the maintenance of peripheral T-cell tolerance. Indeed, early apoptotic bodies deliver a potent immunosuppressive signal to DCs (32). Immature DCs that have internalized apoptotic bodies fail to augment the expression of MHC and costimulatory molecules, secrete less proinflammatory cytokines, induce T-cell apoptosis and decrease T-cell stimulatory function. However, inhibition of costimulation signals seems to be required to prolong allograft survival (32,33). Apoptotic bodies may also negatively impact antibody production. In a murine heart transplantation model, injection of donor apoptotic cells before transplantation decreased the levels of circulating antidonor MHC class I and II alloantibodies compared to untreated recipients (32).
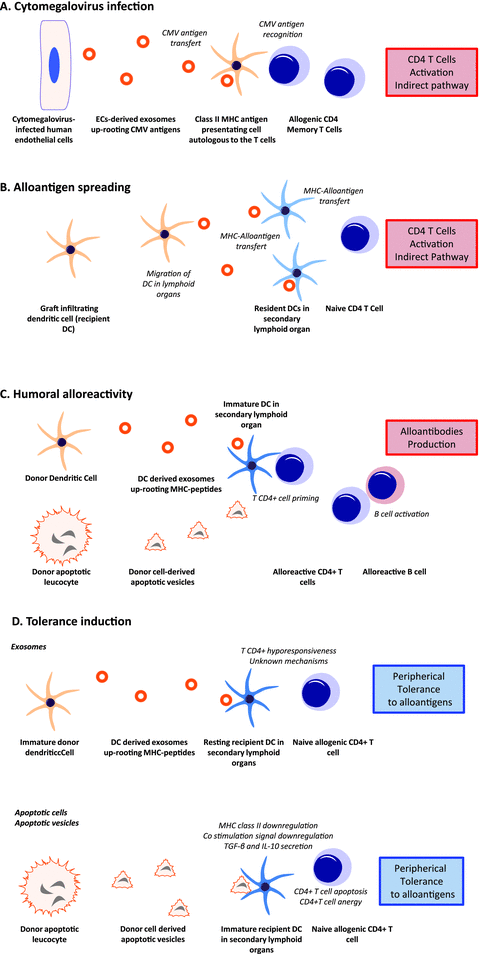
Implications of membrane vesicles secretion in transplantation.
A recent finding by our group suggests that apoptotic cells also emit nanovesicles that are ultrastructurally, biochemically and functionally distinct from apoptotic bodies (34). Apoptotic nanovesicles are smaller than apoptotic bodies, sediment at 100 000 g to 200 000 g and share some common ultrastructural characteristics and protein markers with exosomes (30). Apoptotic nanovesicles, like exosomes, seem to originate from fusion of multivesicular bodies with the cell membrane. Exosomal nanovesicles produced by live DCs can modulate antidonor immune responses by carrying tolerogenic (35) or immunogenic properties, such as amplification and generation of donor-reactive T cells after transplantation (36). EC-derived exosomes are a major source of cytomegalovirus (CMV) antigens recognized by CD4+ memory T-cells (37) and can initiate CMV-specific memory responses of allogeneic CD4 T cells by contact-independent transfer of CMV antigens. Importantly, in these studies, nanovesicles were produced by living or stressed cells, but not by apoptotic cells (Figure 2). Whether apoptotic nanovesicles also regulate immune functions remains to be established.
A step further: large-scale approaches and the characterization of unconventional trafficking
Convincing evidence in heart, renal and lung transplantation has established a central link between the presence and persistence of endothelial apoptosis and the development of maladaptive allograft remodeling (13,15). These data were gathered through meticulous pathology studies in animal models and human transplant samples. Intriguingly, large-scale transcriptomic strategies have not underscored an association between maladaptive transplant remodeling and biomarkers of apoptosis (38). These seemingly contradictory observations probably stem from technical limitations. Activation of apoptotic programmed cell death is regulated at the posttranscriptional level through a series of proteolytic interactions and intracellular translocation events that cannot be captured by transcriptomic strategies. Apoptotic cells are rapidly engulfed by neighboring cells and professional phagocytes, resulting in their rapid disposal and loss of intracellular apoptotic protein fingerprints. It should come as no surprise that apoptosis effectors, such as caspases and endonucleases, have failed to be identified by transcriptomics.
The characterization of soluble mediators released by apoptotic cells likely represents a more reliable means of tracking the consequences of apoptosis in vivo. In support of this assertion, central components of the endothelial apoptotic secretome, LG3 and CTGF, have been associated with allograft rejection and pathological remodeling in human allograft transplants (20,23). Unbiased proteomic approaches to urine samples from renal transplant patients have identified LG3 as a marker of chronic allograft rejection in renal transplant patients (39). Elevated serum LG3 levels were also found in renal transplant patients with acute vascular rejection, compared to tubulointerstitial Banff grade I rejection and normal allografts (23). Collectively, these results indicate that various biomarkers of allograft fibrogenesis are components of the apoptotic molecular legacy, supporting the contention that apoptosis is an important regulator of allograft remodeling.
Conclusion
Dying cells act as key regulators of tissue remodeling and immune system modulation. In transplantation, various stressors, present at the time of organ retrieval, transplantation and in association with rejection episodes, can significantly increase the apoptotic load of allografts. A better understanding of the elaborate and unconventional communication network that dying cells weave into their microenvironment is emerging. The molecular legacy of apoptotic cells controls pathways central to leukocyte trafficking, ECM proteolysis, tissue repair, fibrogenesis and immunity. Tracking death signals will undoubtedly remain a lively field of investigation in transplantation.
Figure 1 summarizes mediators that released by apoptotic ECs and of importance in vascular remodelling. ECs release chemokines and lipid mediators (MCP-1, lysophosphatidylcholine) and express adhesion molecules that will recruit mononuclear leucocytes, favoring diapedesis and intimal accumulation. Apoptotic ECs also release CTGF, EGF and cathepsin L in a caspase 3-dependent manner. The latter favors ECM proteolysis and production of a truncated fragment of perlecan, LG3, which favors neointima formation. Apoptotic ECs also delivers antiapoptotic signals to vascular smooth muscle cell (VSMC) by translationally controlled tumor protein (TCTP) bearing nanovesicles. Chronic production of these mediators could lead to maladaptative vascular remodeling and fibrosis characteristic of chronic TV. EC; VSMC; ECM; EGF; CTGF; TCTP.
A. Exosomes secreted from infected ECs and B cells may transfer viral antigens (CMV) and EBV-derived microRNAs, which can in turn interact with the recipients’ immune system. B, C. Exosomes secreted from ECs or immune cells may transfer antigen and/or peptide—MHC complex to DCs leading to indirect antigen presentation, T CD4+ cells activation, production of alloantibodies and antigen spreading. D. Exosomes secreted from donor apoptotic cells or immune cells may promote tolerance to alloantigens. DC; EC; VSMC.
Acknowledgments
This work was supported by research grants from the Canadian Institutes of Health Research (MOP-15547 and MOP-89869), Fonds de la recherche en santé du Québec (FRSQ, Transdisciplinary Research Group on Predictors of Rejection), Fondation J.-L. Léveseque to MJH and the Shire Chair in Nephrology, Transplantation and Renal Regeneration of Université de Montréal.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.