The Emerging Role of Brazil in Clinical Trial Conduct for Transplantation
Abstract
Brazil is a country with over 190 000 000 inhabitants and a health system composed of a large public, government managed system. Between 1999 and 2010 the number of deceased donors increased by 161%, from 3.8 to 9.9 pmp, and the number of solid organ transplants increased by 121%, from 2891 to 6402. This growth was a consequence of the creation of a well-organized national transplant program. Government funding, decentralization and educational investment in transplant coordinators and related professional were decisive. In 2009 Brazil was the second largest country in the absolute number of kidney transplants (n = 4259). There are significant region disparities in performance which are mainly due to the development status. Improvements in transplant and research regulations resulted in an increasing participation of Brazilian transplant centers in multicenter trials, reaching over 44 studies during the last 11 years. Brazilian centers have been involved in clinical trials using everolimus, sirolimus, fingolimod, mycophenolate mofetyl, mycophenolate sodium, tacrolimus modified-release, sotrastaurin, belatacept, JAK3 inhibitor CP690,550 and valganciclovir. The still increasing number of transplants performed every year along with more efficient regulatory and sanitary analysis, organized clinical research programs and reduction in region performance disparities will eventually increase even more the participation of Brazil in trials worldwide.
Abbreviations:
-
- ABTO
-
- Brazilian Society for Organ and Tissue Transplantation
-
- ANVISA
-
- National health surveillance agency
-
- CEP
-
- Committee for ethics in research
-
- CIHDOTT
-
- Intra-hospital committees for the donation of organs and tissue for transplantation
-
- CNCDO
-
- Centrals for notification, procurement and distribution of organs
-
- CNNCDO
-
- National center for notification, procurement and distribution of organs
-
- CNS
-
- National Health Council
-
- CONEP
-
- National committee for ethics in research
-
- GDP
-
- Gross domestic product
-
- HDI
-
- Human development index
-
- HLA
-
- Human leukocytes antigen
-
- pmp
-
- Per million population
-
- PPP
-
- Purchasing power parity
-
- PRA
-
- Panel Reactive Antibody
-
- SNT
-
- National Transplant system
-
- SPOT
-
- Organ and tissue procurement services
-
- SUS
-
- Sistema Único de Saude, (Unified Brazilian health care system)
Introduction
Brazil is a continental country with a total area of 8 514 877 km2 divided into 26 states and a federal district where the capital Brazilia is located. In 2010 the population reached 190 732 694 inhabitants living predominantly in urban areas (84%) with a projected life expectancy of 73.17 years (1). Currently, 26.7% of the population is between 0 and 14 years, 66.8% between 15 and 64 years and 6.4% 65 years old and over with 53.7% white, 38.5% mulatto (mixed white and black), 6.2% black and 1.6% of other ethnicities. Brazilian gross domestic product (GDP) at purchasing power parity (PPP) per capita is $10 499 (ranked 76) and the human development index (HDI) is 0.699, ranked 73 in the high human development subgroup (2,3). The Brazilian health system is composed of a large public, government managed system (Sistema Único de Saude, SUS), which serves the majority of the population, and a private sector, managed by health insurance funds and private entrepreneurs. The level of public spending is particularly high in comparison with emerging-market peers. According to the World Health Organization, the total expenditure on health was 8.4% of GDP in 2008, 44% from the government sector.
The Demand
In 2007, 94 282 patients (498 per million population, pmp) were on dialysis, a 56.3% increase compared to 2001. This prevalence is lower compared to that observed in Japan (1797 pmp) and United States (1509 pmp), and Latin American countries such as Chile (773 pmp) and Uruguay (846 pmp) (4). Considering that the prevalence of chronic kidney disease is believed to be higher in developing countries than in the developed world (5), end-stage renal disease may be underdiagnosed and/or undertreated in Brazil (6). In 2009, the number of patients in the waiting list for kidney (34 640), liver (4304), kidney/pancreas (576), heart (305) and lung (161) transplants had grown 44–188% during the previous 7 years (7).
Development of Transplant Programs
Transplant programs were born in academic university institutions. First attempts occurred in 1964 for kidney and in 1968 for heart, liver, intestine and pancreas. The first law regulating the removal and transplantation of tissues, organs and parts was approved in 1968 (8). The development of transplant programs in Brazil was linked to the implementetion of an integrated system for the treament of patients with end-stage kidney diseases. From 1976 to 1986, the number of patients undergoing dialysis increased from 500 to 9000 while the number of kidney transplants increased from 729 to 820. This disproportional growth was due to more attractive reimbursements and relative procedure simplicity of dialysis over transplantation. In 1986 the Brazilian Association for Organ and Tissue Transplantation (ABTO) was founded and started taking decisive influence on the development of future policies to increase the number of transplants in the country.
In 1987 the government created an integrated system for the treatment of patients with end-stage chronic kidney diseases, later expanded with the creation of an integrated system of high complexity procedures to regulate and to define standards for accreditation and reimbursement of kidney, liver, heart, lung, pancreas and bone marrow transplantation. From 1988 to 1993 the deceased donor rate increased from less than 1–3 donors/pmp/year. Nevertheless, the proportion of kidney transplant patients compared to those receiving dialysis remained stable, 8% in 1981 and 6% in 1995. The main factor accounting for the failure of these initial federal policies was the lack of mechanisms that would allow the integration of available resources. It was necessary to (1) develop a national policy to improve the regulation and funding of organ procurement organizations and transplant centers; (2) to increase the detection and maintenance of potential organ donors and (3) to built transparency of organ allocation and confidence on the therapy.
In 1997 the National Transplant System (SNT) was created to coordinate and develop the process of recovery and distribution of tissues, organs and parts taken from the human body for therapeutic purposes (9). SNT is an integrated and decentralized system composed by the National Center for Notification, Procurement and Distribution of Organs (CNNCDO), located at the Brasilia airport, and by state Centrals for Notification, Procurement and Distribution of Organs (CNCDO) who organize organ procurement, allocation and waiting lists. Organ and tissue procurement services (SPOT) and regional hospitals coordinate the procedures of organ procurement and recovery. Further improvement occurred between 2001 and 2009, with the creation of 561 Intra-Hospital Committees for the Donation of Organs and Tissues for Transplantation (CIHDOTT) in state hospitals. These units strengthened the contact between these hospitals, SPOTs and CNCDOs, increasing the number of notifications of potential donors, improving donor maintenance and facilitating recovery of organs. Educational programs including training courses for transplant coordinators (10) and regional meetings with intensive care unit professionals, supported by ABTO and sponsored by SNT, were critical to the observed increased in the number of notifications of potential donors (8). Involvement of the society included the creation of nongovernmental organizations, the institution of the national day of organ and tissue donation (September 27th) with annual campaigns in each state during this week supported by regional offices of the ABTO. Organ donation and transplantation was included in teaching programs of several schools of medicine and training and postgraduation courses have also been prepared (11).
In 2009 updated technical regulation of the SNT was approved. Currently, informed consent is required for recovery of deceased donor organs after a short period, from 1997 to 2001, of ineffective and perhaps harmful use of presumed consent form. Deceased donor organ allocation is based on regional waiting lists. Time on the waiting list and medical urgency are used for organ allocation. For kidneys, the allocation system is primarily based on HLA matching with extra points given according to time on dialysis, and to pediatric, sensitized (>80% PRA) or diabetic patients. All organs from donor younger than 18 years are primarily allocated to the pediatric population. Kidneys from living related (up to 4th degree relatives) and spouses are allowed but severe restriction is posed to living unrelated donation, requiring approval by local ethics committee and judicial authorization.
Between 1999 and 2010 the number of potential donors increased 99%, from 18.3 pmp to 36.4 pmp. The number of actual donors increased 161%, from 3.8 pmp to 9.9 pmp (Figure 1A). In 1999 the country performed 2891 solid organ transplants, including 109 hearts, 363 livers, 8 pancreas, 17 lungs and 2394 kidneys (14.6 pmp). In 2010, the total number of organ transplants was 6402, including 166 hearts, 1413 livers, 133 pancreas, 60 lungs and 4630 kidneys (22.4 pmp) (12). These figures represent a 121% increase over 11 years, roughly 10% per year. The number of kidney transplants increased 93% in the same period, almost exclusively due to the increase in the number of transplant with deceased donors. While in 1999 the proportion of living versus deceased donor kidney transplants was 58–42%, by 2010 this proportion was 35–65%, respectively (Figure 1B) (12). A similar increase (85%) was observed in the number of kidney transplants performed in the pediatric population from 1999 (n = 162) to 2010 (n = 301). Of note, only 2.4% of the total number of kidney transplants was from living unrelated donors. The growth of the numbers of liver transplants was significantly higher in the same period (289%).
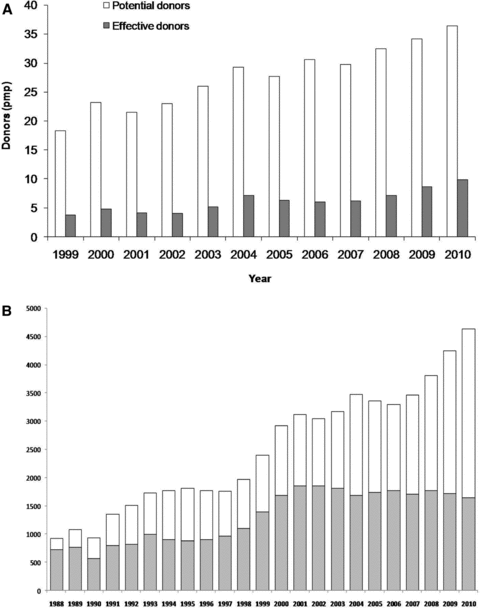
Number of potential and effective deceased donors between 1999 and 2010 (A) and number of living and deceased donor kidney transplants between 1988 and 2010 (B).
Based on reported data to the International Registry of Organ Donation and Transplantation (http://tpm.org/), Brazil was ranked 2nd in absolute number of kidney transplants in 2009 (Figure 2A). Nevertheless, the 22.4 kidneys transplants pmp is below the average of the majority of developed countries (Figure 2B). Interestingly, Brazil would be the leading country if the number of kidney transplants was adjusted by annual GDP per capita (0.42 kidney transplants per US$ GDP per capita) followed by United States (0.37 kidney transplants per US$ GDP per capita). Nevertheless, the gap between supply and demand is very high. In 2009 11 040 new patients with chronic kidney disease were included in the waiting list but only 4259 kidney transplants (38%) were performed.
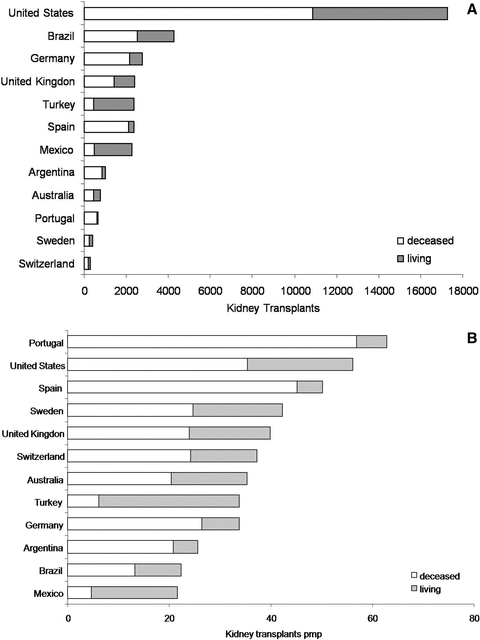
Absolute (A) and adjusted per million population (B) number of living and deceased donor kidney transplants in 2009 in selected countries.
There are obvious performance disparities within the country that are related mainly to differences in demographic density, GDP and level of development (Table 1). In 2010, Brazil had 9.9 deceased donors pmp, ranging from 1.9 pmp in the North to 12.6 pmp in the South-East region. Of 138 active kidney transplant centers, 73% were in the South-East and South regions and only 19 did more than 50 kidney transplants this year. The overall number of kidney transplants was 24.1 pmp, ranging from 6.1 pmp in the North to 31.2 pmp in the South region (12,13). Moreover, there are also disparities within the same region, where the level of organization is perhaps the key factor accounting for the observed differences. For example, in the most developed region (South-East), the rate of kidney transplants was 66 pmp in São Paulo State (21.1 pmp living and 44.9 pmp deceased donors) and 15.8 pmp in Rio de Janeiro State (7.2 pmp living and 8.6 pmp deceased donors).
| North | Center-West | North-East | South | South-East | |
|---|---|---|---|---|---|
| Number of states | 7 | 3 | 9 | 3 | 4 |
| Area (km2) | 3 869 637 | 1 606 371 | 1 558 196 | 576 410 | 924 511 |
| Population (%) | 8 | 7 | 28 | 14 | 43 |
| Population density (n/km2) | 4 | 8 | 32 | 48 | 84 |
| GDP (%) | 6 | 8 | 13 | 17 | 56 |
| CNCDO (n) | 5 | 3 | 9 | 5 | 18 |
| CHIDOTT (n) | 18 | 50 | 66 | 109 | 318 |
| Immunogenetic laboratories (n) | 1 | 4 | 6 | 11 | 23 |
| Effective donors (pmp)* | 1.9 | 9.3 | 5.5 | 11.5 | 12.6 |
| Kidney transplants—living (pmp)* | 5.2 | 8.0 | 3.3 | 10.4 | 9.4 |
| Kidney transplants—deceased (pmp)* | 0.8 | 11.6 | 7.8 | 20.8 | 18.7 |
| Kidney transplants—total (pmp)* | 6.1 | 19.6 | 11.4 | 31.2 | 28.1 |
| Dialysis units, n = 594 (%)# | 3 | 9 | 15 | 24 | 49 |
| Patients on dialysis, n = 53,816 (%)# | 3 | 6 | 22 | 17 | 52 |
| Kidney transplant centers, n = 235 (%)* | 2 | 6 | 19 | 22 | 51 |
| Active kidney transplant centers, n = 138 (%)* | 2 | 5 | 20 | 20 | 53 |
| Kidney transplant patients, n = (%)* | 1 | 4 | 14 | 19 | 62 |
| Society members, n = 1058 (%) | 3 | 4 | 19 | 15 | 59 |
| Indexed publications, n = 409 (%) | 0 | 1 | 3 | 16 | 80 |
- CNCDO = Central for Notification, Procurement and Distribution of Organs; CHIDOTT = Intra-Hospital Committees for the Donation of Organs and Tissues for Transplantation; *data from 2010; #data from 2009.
Regulation of Clinical Research
In 1996 the National Health Council (Conselho Nacional de Saude, CNS) approved guidelines and regulating norms for research involving human subjects in accordance with international standards and with the provisions of the Constitution and related Brazilian legislation. The National Committee for Ethics in Research (Comissão Nacional de Ética em Pesquisa, CONEP), an independent collegiate body, was created to define ethical standards, guidelines and norms for research conduct, to promote the creation and registration of local Committees for Ethics in Research (Comitê de Ética em Pesquisa, CEP) and to monitor all ethical aspects of research involving human subjects. Research involving human subjects must be submitted to local CEPs for evaluation within 30 days. Protocols involving special populations, human genetics and reproduction, pharmaceutical products and multicenter international participation are forwarded to CONEP for further evaluation within 60 days (14). The National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária, ANVISA) also reviews and follows the technical, scientific and pharmacovigilance aspects of the research, authorizing importation of research materials, including the experimental drug and initiation of the trial (15).
Clinical Trials in Brazil
In 1987 the first national phase IV multicenter trial was conducted after registration of the oil formulation of cyclosporine. This study was followed by other phase IV multicenter national studies after the registration of mycophenolate mofetyl and basiliximab in 1998. The first phase III international multicenter clinical trial in kidney transplant recipients with participation of two Brazilian centers occurred in 1999. After this initial experience the number of centers and patients included in national and international trials in kidney transplant recipients has increased substantially reaching more than 44 studies and 2903 enrolled patients. Brazilian centers have participated in clinical trials using everolimus (16–18), fingolimod (19–23), sirolimus (24–26), mycophenolate mofetyl (27–31), mycophenolate sodium (32–34), tacrolimus (35,36), tacrolimus modified release (37,38), valganciclovir (39), belatacept (40), sotrastaurin (clinicaltrials.gov identifiers NCT00504543 and NCT01064791) and the JAK3 inhibitor CP690 550 (clinicaltrials.gov identifier NCT00483756).
Among several factor accounting for the emerging role of Brazilian transplant centers in clinical trial conduct two were decisive. First, the regulation of transplant and research programs and second, the growing number of transplants performed every year. This increased participation in transplant trials has created a positive feedback mechanism for transplant centers, country, global organizations and the transplant community. Transplant centers developed expertise and invested in training to meet international standards in conducting clinical trials. International interaction and exchange information has been applied to routine clinical assistance. This increased level of organization has been associated with steady improvement in performance and transplant outcomes (41). Protocol design started to take into account wider geographical differences allowing a more reliable extrapolation of research findings to local patient populations.
From pharmaceutical organizations perspective, development of drugs in emerging areas with proper research legislation was initially more cost-effective. Although regulatory and sanitary approval usually takes 6 months, transplant centers have improved their capabilities (infrastructure and human resources to conduct a clinical trial) and have consistently enrolled and started surpassing number of patients previously committed to global organizations, generating high-quality data. This has been confirmed by several independent audits conducted by sponsors and recent FDA inspections in two research centers. Locally, pharmaceutical organizations have developed better relationship with local Health Authorities (ANVISA) and Ethics Committees (CONEP/CEP) through better regulatory packages elaborated from these sites with a direct impact on approval timeliness. Moreover, by being consistently reliable in this therapeutic area, Brazilian credibility has also increased. In this scenario, not only high-quality data are generated from Brazil to global corporations collaborating to better quality and faster dossiers to global Health Authorities but also have helped pharmaceutical organizations achieve individual trials and regulatory goals without having to rely exclusively on saturated regions for clinical trials such as Eastern Europe and United States. Finally, local drug registration processes were facilitated.
There are also substantial benefits for the patients. Disparities in patient demographics yielding different sensitivity to particular drug or drug regimen allow further optimization of immunosuppressive strategies, anticipating safety issues before registration of the tested drug and initial use in large-scale nonselected population. As we move toward worldwide unified drug development and registration process and to personalized medicine, the participation of populations with ethnical and geographical differences in clinical trials is important, even more when one take into account the high variability in genetic determined immune responses and drug sensitivity, and the interaction of the type of immunosuppression with regional endemic diseases and available prophylactic strategies.
For the global transplant community, the emergence of well-organized and growing transplant programs in countries such as Brazil, Argentine, Turkey and Korea is an opportunity to exchange information in many different aspects of transplant policies, practice and performances, opening opportunities for future collaborations and improvement of transplant research and clinical practice worldwide. These transplant programs are also committed with all ethical aspects related to transplantation, certainly contributing to curtail organ trafficking and transplant tourism.
Challenges and Future Directions
After the creation of the SNT in 1997 and the development of an integrated national system, the Brazilian transplant program has experienced a substantial growth in numbers, access and performance. Government funding increased from US$ 46 million dollars in 1998 to 583 million dollars in 2009. Nevertheless, the challenges ahead are substantial. The gap between the estimated need and actual number of transplants is still high and increasing. For example, the estimated need for kidney transplants is 60 pmp per year but we are currently performing only 38% of this demand. For liver, heart and lungs the gap is even bigger. From 2001 to 2009, the waiting list grew 44% for kidneys up to 182% for lungs.
In 2008 ABTO proposed measures to increase the number of deceased donors to 20 pmp by 2018 (42). We need to increase the rate of notification of potential donors (currently 36.5 pmp) and the proportion of notified potential donors who become effective donors (currently 25%). Faster identification and better maintenance are fundamental because family refusal has been stable around 26% during the last years. The society also proposed to increase the number of kidney transplants with living donor from the currently 9.1–20 pmp within the next 10 years. It is also time for a critical evaluation of the transplant programs. In this direction ABTO started organizing a registry collecting key organ transplant outcome data. This analysis will be important to define future directions and to guide proper investment in a government-based program with restricted budget.
To achieve these goals we will need to increase government funding in all geographic regions. Investment in educational programs will be crucial to capacitate more professionals to increase the notification of potential donors. There is an urgent need of trained specialists, including surgeons, nephrologists, immunogeneticists and pathologists. Also, we will need to increase the number of hospital beds to accommodate not only the increasing number of new transplants but also to treat the increasing cumulative number of patients on late follow-up, who frequently show complex and expensive treatments.
The initial successful experience in clinical research also needs to be expanded. Similarly to the SNT, research regulation need to be more efficient and decentralized. Investment in qualified professionals is fundamental. It is also crucial to create and involve more research centers. While Brazilian participation has been limited to less than five centers and concentrated mainly in kidney transplantation, this scenario is expected to change as the number of transplants increases and many more centers are organizing clinical research units, especially with the incentive of the Brazilian government agencies. Initial experiences with national multicenter trials are encouraging.
The transplant and research programs in Brazil are still in early ages but are very promising. Substantial investment in infra-structure and professionals along with increased efficiency will be needed to accommodate the anticipate increasing demand in the coming years.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation.
Tedesco Silva H. Jr. has received consulting and travel honoraria and research grants from Novartis, Roche, Pfizer, Wyeth, Bristol Meyer Squibb, Astelas and Jansen-Cilag.
Felipe C.R. has nothing to disclose.
Abbud-Filho M. has received consulting and research grants from Novartis, Wyeth, and Jansen-Cilag.
Garcia V. has received travel honoraria from Novartis, Roche, Wyeth and Jansen-Cilag, and research grants from Novartis, Roche, Pfizer, Wyeth, Bristol Meyer Squibb, Astelas and Jansen-Cilag.
Medina-Pestana J.O. has received consulting and travel honoraria and research grants from Novartis, Roche, Pfizer, Wyeth, Bristol Meyer Squibb, Astelas and Jansen-Cilag.