A Complete Treatment of Adult Living Donor Liver Transplantation: A Review of Surgical Technique and Current Challenges to Expand Indication of Patients
Abstract
The growing disparity between the number of liver transplant candidates and the supply of deceased donor organs has motivated the development of living donor liver transplantation (LDLT). Over the last two decades, the operation has been markedly improved by innovations rendering modern results comparable with those of deceased donor liver transplantation (DDLT). However, there remains room for further innovation, particularly in adult living donor liver transplantation (ALDLT). Unlike whole-size DDLT and pediatric LDLT, size-mismatching between ALDLT graft and recipient body weight and changing dynamics of posttransplant allograft regeneration have remained major challenges. A better understanding of the complex surgical anatomy and physiologic differences of ALDLT helps avoid small-for-size graft syndrome, graft congestion from outflow obstruction and graft hypoperfusion from portal flow steal. ALDLT for high-urgency patients (Model for End-Stage Liver Disease score >30) can achieve results comparable to DDLT in high volume centers. Size limitations of partial grafts and donor safety issues can be overcome with dual grafts and modified right-lobe grafts that preserve the donor's middle hepatic vein trunk. Extended application of LDLT for unresectable hepatocellular carcinoma above Milan criteria is an optional strategy at the cost of slightly compromised survival. ABO-blood group incompatibility obstacles have been broken down by introducing a paired donor exchange program and refined peri-operative management of ABO-incompatible ALDLT. This review focuses on recent innovations of surgical techniques, safe donor selection, current strategies to expand ALDLT with broadened patient selection criteria and important aspects of teamwork required for success.
Abbreviations
-
- 3D
-
- three-dimensional
-
- ABO-c
-
- ABO-compatible
-
- ABO-i
-
- ABO-incompatible
-
- ACLF
-
- acute-on-chronic liver failure
-
- ALDLT
-
- adult living donor liver transplantation
-
- AMR
-
- antibody-mediated rejection
-
- AR
-
- acute rejection
-
- AS
-
- anterior sector
-
- BD
-
- bile duct
-
- BMI
-
- body mass index
-
- BS
-
- biliary stricture
-
- CT
-
- computed tomography
-
- D-D
-
- duct-to-duct
-
- DDLT
-
- deceased donor liver transplantation
-
- DIHBS
-
- diffuse intrahepatic biliary stricture
-
- ECMO
-
- extracorporeal membrane oxygenation
-
- ERL
-
- extended right-lobe
-
- FHF
-
- fulminant hepatic failure
-
- GRWR
-
- graft-recipient weight ratio
-
- GSV
-
- great saphenous vein
-
- GV/SLV
-
- graft volume/standard liver volume
-
- HAT
-
- hepatic artery thrombosis
-
- HCC
-
- hepatocellular carcinoma
-
- H-J
-
- hepaticojejunostomy
-
- HPCS
-
- hemi-portocaval shunt
-
- HV
-
- hepatic vein
-
- ICG R15
-
- indocyanine green retention rate at 15 min
-
- IOCP
-
- intraoperative cineportography
-
- IOUS
-
- intraoperative doppler ultrasound
-
- IVC
-
- inferior vena cava
-
- LDLT
-
- living donor liver transplantation
-
- LHV
-
- left hepatic vein
-
- LIT
-
- local infusion therapy
-
- LL
-
- left-lobe
-
- MELD
-
- Model for End-Stage Liver Disease
-
- MHV
-
- middle hepatic vein
-
- MRL
-
- modified right-lobe
-
- PE
-
- plasmapheresis
-
- PNF
-
- primary nonfunction
-
- PS
-
- posterior sector
-
- P-S
-
- porto-systemic
-
- PTFE
-
- polytetrafluoroethylene
-
- PV
-
- portal vein
-
- PVF
-
- portal venous flow
-
- PVP
-
- portal venous pressure
-
- RBT
-
- rubber-band tagging
-
- RHV
-
- right hepatic vein
-
- RL
-
- right-lobe
-
- RLV
-
- remnant liver volume
-
- SFS
-
- small-for-size
-
- SRHV
-
- short right hepatic vein
-
- TLV
-
- total liver volume
Introduction
A major concern in adult living donor liver transplantation (ALDLT) is determining the graft size that can be safely removed from donors. Previously, a left-lobe (LL) was the major graft used in ALDLT. However, the frequent extremes of graft-size insufficiency in LL living donor liver transplantation (LDLT) represented a greater risk of small-for-size (SFS) graft syndrome. The development of right-lobe (RL) LDLT has significantly increased graft supply since RL grafts enable donation from donors with smaller in body size relative to recipients, and has markedly increased ALDLT performance throughout the world 1. The surgical technique of deceased donor liver transplantation (DDLT), and pediatric and adult LL LDLT has been standardized; however, RL LDLT is often technically challenging for its multiple biliary and complex hepatic vein (HV) reconstructions. The incidence of biliary complications remains high after LDLT 2, and acceptance criteria of RL grafts related to the multiplicity of ducts varies among transplant centers 3. Outflow occlusion and congestion remain potential hurdles 4. Despite these technical challenges, ongoing innovations have advanced RL LDLT to allow for results comparable to DDLT for many advanced indications. Attention to middle hepatic vein (MHV) reconstruction has been a critical factor 5. Extracorporeal membrane oxygenation (ECMO) and nutritional support through a feeding jejunostomy have been used for critically ill patients. Complete occlusion of the portal vein (PV) is included as an indication of LDLT by application of intraoperative cineportography (IOCP) and various innovative jump graft anastomoses. ABO-incompatible (ABO-i) LDLT comprises about 25% of modern ALDLTs with comparable results to ABO-compatible (ABO-c) ALDLT. With a high incidence of hepatitis B-related hepatocellular carcinoma (HCC) and a low deceased donor organ donation rate, HCC with or without hepatic decompensation constitutes nearly half the ALDLTs in many Asian centers. Herein, I present my views on technical aspects of ALDLT, with particular focus on factors ensuring technical success.
Early Development
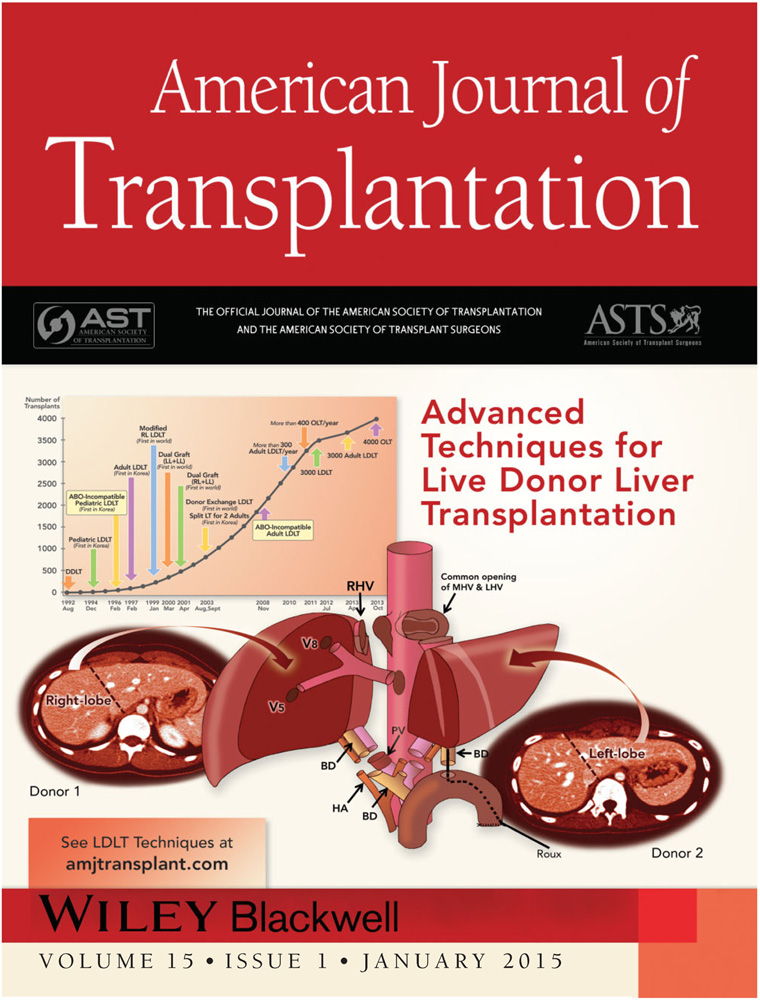
After the first successful ALDLTs with LL and subsequently RL grafts in 1997 at the Asan Medical Center, the number of ALDLTs increased remarkably, driven by the shortage of deceased donors, the high prevalence of cirrhosis from hepatitis B and HCC, and more recently, the application of ABO-i ALDLT 6 (Figure 1). Immediately after our first successful RL LDLT, we faced the obstacle of anterior sector (AS) congestion of RL grafts, and in 1998, we initiated reconstruction of the MHV tributaries for modified RL grafts (Figure 1). Dual LDLT was developed to address graft-size insufficiency and promote donor safety 7. From 2000 to 2012, 346 dual LDLTs with various combinations of liver grafts were performed with comparable results to RL LDLTs. In 2003, a donor exchange program for ALDLT was initiated to avoid potential complications associated with ABO-i LDLT and to expand the living donor pool, but with limited success. In November 2008, ABO-i ALDLT was launched cautiously with dual transplants, by a mixture of ABO-c and ABO-i liver grafts.
To date, 250 ABO-i ALDLTs confined to low Model for End-Stage Liver Disease (MELD) patients have been performed with a 1-year graft survival rate of 95.8%. The proportion of HCC patients receiving LDLT has increased rapidly from 18.7% to 50.3% during the last 10 years. Salvage LDLT in previously hepatectomized patients for HCC is increasingly performed with acceptable outcomes, but with higher perioperative morbidities compared to primary LDLT. In 2010 and 2011, 301 and 300 ALDLTs were performed, respectively (Table 1). Liver graft type for ALDLT was selected to meet the metabolic demands of the patient, with at least 0.7 graft-recipient weight ratio (GRWR) in low-MELD patients and near 1.0 in high-MELD patients. Unimpeded hepatic venous outflow drainage is crucial to maximize hepatocyte function. The most common graft type for ALDLT has been a modified RL (MRL; 83.5%), then dual (7.8%), extended RL (ERL; 5.2%), RL without MHV reconstruction (2.3%) and LL grafts (1.2%) (Table 1). Mean GRWR of LL grafts was 0.85 at its lowest, mainly selected for donor safety.
| Number of ALDLT (year 2010/2011) | 601 ALDLT (301/300) | |
| Age (mean, range) | 51.9 years (range: 19–72) | |
| Sex (male/female) | Male: 458, female: 143 | |
| MELD scores | 16.6 ± 9.2 (range: 5–52) | |
| CTP scores | 8.2 ± 2.4 (range: 5–15) | |
| Primary disease of adult recipients | ||
| HBV | 411 (68.38%) | |
| Alcohol | 73 (12.14%) | |
| HCV | 42 (6.98%) | |
| Cryptogenic | 22 (3.66%) | |
| Fulminant hepatic failure | 22 (3.66%) | |
| Autoimmune disease | 10 (1.66%) | |
| Wilson's disease | 7 (1.16%) | |
| Other1 | 141 (2.32%) | |
| Presence of hepatocellular carcinoma | 296 (49.4%) | |
| Salvage LDLT | 36 (12.2%) | |
| ABO incompatible LDLT | 72 (12.0%) | |
| Urgent LDLT | 86 (14.3%) | |
| Fulminant hepatic failure | 22 (25.6%) | |
| Acute-on-chronic liver failure | 64 (74.4%) | |
| GRWR% (mean, range) | 1.13% (0.68–2.43) | |
| Graft types | Number | (Mean GRWR%) |
| Right-lobe | 14 (2.32%) | (1.26%) |
| Modified right-lobe | 502 (83.52%) | (1.15%) |
| Extended right-lobe | 31 (5.15%) | (0.92%) |
| Dual graft | 47 (7.82%) | (1.02%) |
| Left-lobe | 7 (1.16%) | (0.85%) |
| Use of artificial vascular graft (PTFE/Dacron) | 13 | |
| Replacement of inferior vena cava | 6 | |
| Jumping graft of portal vein | 7 | |
| In-hospital mortality | 18 (3.1%) | |
| Donors outcome | ||
| Major morbidity | 5/646 (0.8%) | |
| Mortality | None | |
- CTP, Child-Turcotte-Pugh; GRWR, graft-recipient weight ratio; HBV, hepatitis B virus; HCV, hepatitis C virus; LDLT, living donor liver transplantation; MELD, Model for End-Stage Liver Disease; PTFE, polytetrafluoroethylene.
- 1 Polycystic liver disease 4, cholangiocarcinoma 2, secondary biliary cirrhosis 2, hepatic epithelioid hemangioendothelioma 2, Budd–Chiari syndrome 1, portal biliopathy 1, citrullinemia 1, glycogen storage disease 1.
In patients with complete PV obliteration, we have changed the PV conduit used for a jump graft from the superior or inferior mesenteric veins, left renal vein, coronary vein or sizable retroperitoneal collaterals from fresh deceased donor vessel grafts, to ringed polytetrafluoroethylene (PTFE) grafts in order to proceed promptly to LDLT in urgent situations (Table 1). None of the patients with 13 inferior vena cava (IVC) replacements and 12 PV jump grafts showed infection or thrombotic complication with use of these artificial vascular grafts.
As of April 2013, there has been no donor mortality among 3000 ALDLT and 224 pediatric LDLTs. Since 2002, perioperative annual major donor morbidity rate (Clavien classification grade II and III) declined from 6.7% to 1.3%. The 1- and 5-year survival rates of ALDLT patients were 93.5% and 83.2%, respectively, including 84.4% 1-year survival of acute-on-chronic liver failure (ACLF) patients. The most common causes of patient loss has been infection, technical failure and early recurrence of HCC. ALDLT is often performed under less-than-ideal conditions, such as small graft volume, short and small vascular pedicles or difficult biliary reconstruction. The safety margin is small in both recipients and donors.
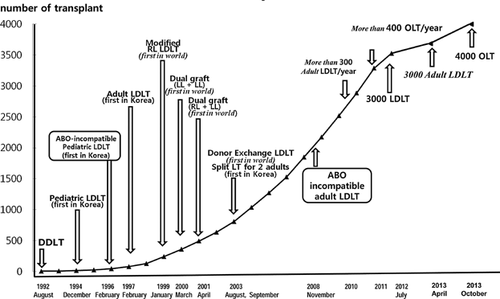
Over time it has become apparent that there are four essential requirements for technically successful LDLT: adequate graft volume to avoid SFS graft syndrome; good venous outflow to prevent congestion injury; sufficient portal inflow for rapid liver graft regeneration; and secure bile duct (BD) anastomosis to prevent biliary leak and stricture (Figure 2). Every step of these procedures must be planned carefully, performed meticulously and tailored to the individual patient. Continuous postoperative surveillance is important after RL and dual LDLT because venous outflow and biliary complications more often develop in these grafts than in other types of liver transplantation. An expert team of intervention radiologists and endoscopists, combined with the dedication of transplant surgical personnel with institutional support, is the fundamental basis for the success of this challenging procedure.
Review of Surgical Technique
Safe donor selection criteria
Since the first ALDLT, the performance of a major hepatectomy on a healthy individual with no medical indication has been viewed with caution and skepticism. Indeed, ensuring the donor's safety and long-term well-being has become the prime imperative for any LDLT program. The practice of ALDLT has been self-perpetuating because as outcomes have improved, more people have become aware of the feasibility of this treatment option; this then encourages donors to volunteer. Donor risk, however, has not yet been eliminated completely, and the donor risk remains the prime directive. Therefore, strict donor selection criteria are required 8. Potential donors for RL hepatectomy are generally confined to healthy volunteers under 55 years of age. Older donors (>50 years) have an increased risk of occult medical diseases, and their livers have reduced regenerative capacity 9.
- ≤35 years and no fatty change
- 30% remnant liver volume (RLV): Acceptable
- ≤35 years and ≤15% fatty change
- 30–35% RLV: Acceptable
- ≤35 years and ≤30% fatty change
- ≥35% RLV: Acceptable
- 35–55 years and ≤15% fatty change
- >35% RLV: Acceptable
RLV should be fully functioning without venous congestion. The minimally accepted RLV in RL donors should be individualized by donor age and the degree of steatosis. Absolute exclusion criteria for RL liver donations are RLV <30% of total liver volume (TLV), >30% macrosteatosis in the preoperative liver biopsy, and a BMI >30 kg/m2 in age >30 years. Liver insufficiency due to an inappropriately voluminous resection is the main cause of death or need for liver transplantation in donors. Besides preoperative volumetric measurement of the RLV, estimation of liver quality by preoperative US-guided liver biopsy must be assessed prior to donation if the BMI is >25, the aspartate transaminase/alanine transaminase are >30 IU/L, the indocyanine green retention rate at 15 min (ICG R15) is >20%, the computed tomography (CT)/magnetic resonance is suggestive of steatosis, the donor age is >35 years or the RLV is calculated to be marginal even with normal liver function. RL hepatectomy carries an estimated mortality risk of 0.5% and still carries a significant morbidity rate, even in very experienced hands. In our institute, the high donor morbidity rate after RL hepatectomy during the learning period has been substantially reduced with the accumulation of experiences (>200 RL LDLT). In the 215 RL hepatectomies in 1997–2001, the following complications arose: 6 patients with intra-abdominal bleeding; 2 PV stenosis and thrombosis after segmental resection/end-to-end anastomosis in type III PV anatomical anomaly in order to obtain single PV orifice of liver graft; 1 PV kinking due to redundancy by extensive dissection; 4 hepatic duct strictures by inadequate vascular supply resulting from unnecessary wide hilar dissection; 2 intraoperative T-tube insertions for narrowing the ductal confluence resulting from too-intimate transection of right hepatic duct; and 3 intra-abdominal abscesses by cut-surface bile leak. All were successfully treated with interventional ballooning, stenting and embolization, except three reexplorations for hemorrhage. Since 2002, the perioperative major morbidity rate (Clavien classification grades II and III) of RL hepatectomy has declined from 10.7% to 1.9%. From 2005, our acceptable limit of RLV after RL hepatectomy has been lowered to 30% from 35% of TLV. There have been no donor deaths in more 3000 ALDLTs performed. This and the low morbidity rate are likely facilitated by our ongoing practice of HCC resections in cirrhotic livers and advanced hepato-biliary-pancreatic surgery, as well as strict guidelines of safe donor selection criteria.
Donor hepatectomy techniques should be designed with donor safety foremost in mind, but complete liver graft formation is the next concern. Unnecessary extensive hilar dissection and a practice of leaving short vascular stumps to elongate the graft vascular pedicles will result in hepatic duct stricture by arterial ischemia, PV stenosis, transection of common hepatic duct or vena caval stenosis with thrombosis in donors. An incomplete liver graft with short vascular pedicles is readily transformed into a complete liver graft with ductoplasty, circumferential fencing of the PV or HV openings by a recipient's remnant PV or great saphenous vein (GSV) at the back table. Currently, laparoscopic donor hepatectomy is a new, challenging field that possesses threatening complications to donors and recipients, especially during BD transection. In our department, totally laparoscopic hepatectomy is confined to lateral sector graft resection only. Recovery of an LL graft is readily carried out through upper midline open incision, and hand-assisted laparoscopic surgery is applied in a quarter of RL hepatectomies with a 10–12 cm subcostal incision, particularly for young unmarried women, as most male donors do not show concern for the limited J-shape incision scar.
It is undeniable that the majority of donors suffer physically and mentally in the first few months after their operation. However, none of them require chronic medical treatment. Our survey demonstrates that most donors consider their recovery complete, and >80% state that they would donate again 10. The median time to return to work and normal social activity is 2 months. Lack of constant vigilance and loosening of acceptance criteria after a few heroic experiences of donor hepatectomy are the major reasons for donor mortality and disabling morbidity. To avoid these disasters, transplant surgeons should maintain the role of gatekeeper in preventing unjustified and risky donor operations.
Adequate graft size and SFS graft
A major limitation for ALDLT is the size of graft that can be safely recovered from a donor. The minimal graft size required to sustain survival of an adult patient is not clearly defined 11, 12. SFS grafts often cannot meet the metabolic demands of the patient, or at times may not even function after implantation. Moreover, the smaller the graft, the less durable it is to all postoperative surgical and medical complications. Despite isolated reports to the contrary, it is generally accepted that a liver graft that provides 40–50% of the patient's graft volume/standard liver volume (GV/SLV) or GRWR 0.8–1 is necessary for patient survival.
Many cases of graft failure are ascribed to SFS graft injury, but we have found that graft size may not be the only critical factor in these failed cases. Outcomes of SFS graft should be viewed considering the interrelationship between graft size, graft quality, MELD score, portal inflow and venous outflow. To improve outcomes, surgeons should be meticulous in eliminating all possible adverse factors. The object is to make all hepatocytes in an SFS graft function immediately after implantation. When optimal graft quality is assured, perfect venous outflow is perhaps the most essential element for success in SFS graft transplantation (Figure 3).
Surgeons now pay meticulous attention to outflow reconstruction and recognition of this critical principle has fostered much better results. For example, with AS drainage and perfect short right hepatic vein (SRHV) reconstruction, the RL graft size in low-MELD patients can be reduced to 35% of GV/SLV or a GRWR of 0.7.
The impact of donor age on survival of patients receiving SFS grafts is well-documented 9. Particularly bad results are seen when GRWR of <0.8 is combined with donor age of >50 years. A similar additive effect is observed when using SFS grafts from donors with steatosis (>30%). Defining a graft as SFS should be done on an individual basis, taking into consideration a number of graft and recipient factors.
- Adequate graft volume
- GRWR >1%
- GRWR >0.8% + Young Donor (<35 years)
- GRWR >0.7% + Young Donor + low MELD (<20)
- Good outflow without congestion is more important than graft volume per se
- Optimal portal inflow should be assured for partial liver graft regeneration
- No/minimal steatosis.
Despite the statistically significant negative impact of SFS grafts on graft function, exceptional successes have been reported. Our experience of successful SFS grafts among 525 ALDLT from February 1997 to August 2003 includes 11 cases of GRWR <0.6, the lowest of which was 0.49 (Table 2).
| No. | Pre-LT bilirubin (mg/dL) | Original disease | Recipient/donor age (year) | Graft | GRWR (%) | Post-LT cholestasis | Post-LT peak bilirubin (mg/dL) | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.1 | HBV-LC | 39/26 | LL | 0.58 | No | – | Alive |
| 2 | 4.3 | HBV-LC | 59/32 | LL | 0.58 | Yes | 32.0 | Alive |
| 3 | 10.0 | HBV-LC | 36/26 | LL | 0.57 | Yes | 33.0 | Alive |
| 4 | 5.3 | HBV-LC | 49/24 | LL | 0.58 | No | – | Alive |
| 5 | 9.5 | HBV-LC | 52/26 | LL + S1 | 0.55 | ? | – | Died (PPH) |
| 6 | 7.1 | HBV-LC | 45/17 | LL | 0.55 | Yes | 18.0 | Alive |
| 7 | 4.8 | HBV-LC | 36/32 | LL | 0.52 | Yes | 17.0 | Died (AR, pancreatitis) |
| 8 | 42.5 | HBV-LC | 31/42 | LL | 0.525 | No | – | Alive |
| 9 | 1.7 | HBV-LC HCC | 42/37 | ERL | 0.56 | No | – | Alive |
| 10 | 4.0 | HBV-LC | 56/25 | LL + S1 | 0.49 | No | – | Alive |
| 11 | 32.8 | FHF | 24/20 | LL + S1 | 0.50 | Yes | 24.0 | Alive |
- AR, acute rejection; ERL, extended right-lobe; FHF, fulminant hepatic failure; GRWR, graft-recipient weight ratio; HBV-LC, hepatitis B cirrhosis; HCC, hepatocellular carcinoma; LL, left-lobe; LT, liver transplantation; PPH, portopulmonary hypertension; S1, caudate lobe.
Because the narrow margin of safety is more pronounced when transplanting SFS grafts into patients with poor clinical status (i.e. ACLF), we confined these very SFS grafts to 10 nonintensive care unit-bound cirrhotic patients and 1 fulminant hepatic failure (FHF) patient. All patients underwent splenic artery ligation for portal flow modulation, and the patient with GRWR of 0.49 underwent left renal vein ligation to interrupt a large spontaneous splenorenal collateral for abolishing postoperative portal flow steal and subsequent graft hypoperfusion. Excluding one early posttransplant death by portopulmonary hypertension, half of the patients developed SFS graft syndrome with prolonged cholestasis. Noticeably, these examples of successful SFS grafts were from young donors, the recipients were also fairly young, and perfect venous outflow was guaranteed in all grafts by retaining MHV. Our strategies to overcome SFS graft syndrome include provision of optimal venous outflow, interruption of portal flow steal with aid of IOCP, modulation of excessive portal inflow by splenic artery ligation, prevention of acute rejection (AR) and use of young donors.
Better understanding of the mechanisms of SFS graft injury in recent years has improved outcome. However, to increase the margin of safety of ALDLT and ensure uniformly good patient outcomes, preoperative selection of liver graft types should still be based on graft size measured by CT volumetry, with recommended of a GRWR of >0.8–1.
Hepatic venous outflow reconstruction
RL LDLT is a technically demanding procedure particularly because of the unique functional anatomic characteristics of venous drainage. Outflow occlusion and congestion lead to suboptimal graft function, graft failure and, in extreme cases, patient death by SFS graft syndrome 4. All venous outflow complications are related to inappropriate technical design of the HV anastomosis and underestimation of the importance of MHV reconstruction. Before recognition of this phenomenon even with a theoretically sufficient liver graft volume, graft function and patient survival were not dramatically improved compared LL grafts. Currently, the major concerns of RL LDLT are focused on donor safety and reconstruction of MHV to avoid congestion injury of AS 13-15. With experience, we have come to recognize that long-lasting patency of the right hepatic vein (RHV) and good SRHV drainage are as important as MHV reconstruction for the integrity of RL graft function. Therefore, we now stress that three anatomical outflow drainage routes should be meticulously reconstructed to make a congestion-free allograft.
First, the AS drainage should be performed by an ERL graft in which the MHV trunk is included in the liver graft, or by an MRL graft in which the venous tributaries of MHV (V5/V8) are connected via interposition vascular grafts to the recipient's HV or vena cava. Second, if multiple SRHVs exist, perfect posterior sector (PS) drainage is better ensured by transforming multiple SRHVs to a common, large opening, instead of individual anastomoses of these veins. Third, a wide, stable RHV reconstruction is doubly assured by venoplasty not only in the recipient's HV (IVC orifice enlargement), but also in the liver graft's intrahepatic venous ostium (RHV caudal angle blunting at back table). These are described in detail below.
Anterior sector AS drainage
Anatomically, the MHV is an important drainage vein of the right-hemiliver in 85% of RL donors. On a donor's preoperative three-dimensional (3D) liver CT, the MHV is the same size as the RHV in 43% of cases, and is bigger than the RHV in 14% of cases 16. In ALDLT, the recipient safety margin is narrow because of frequent graft-size disparity and higher surgical complications owing to multiple biliary and hepatic venous reconstructions. To increase the safety margin and help alleviate graft-size disparity by avoiding AS congestion injury and maximizing graft function, MHV drainage of RL graft must be optimized. Occlusion of the MHV outflow drainage has been a decisive factor leading to recipient morbidity and in-hospital mortality. MHV drainage is accomplished by two methods of donor hepatectomy: ERL and MRL grafts. From the viewpoint of donor safety, ERL grafts increase donor risk because the donor's remnant LL does not possess the MHV, and varying degrees of medial sector congestion injury develop in essentially all donors 17. Although ERL grafts are the perfect design for outflow drainage of AS, MRL grafts provide similar functioning liver mass if sizable MHV tributaries are preserved by precise scrutiny of the anatomy of V5 and V8, based on the donor's 3D CT, and the meticulous back-table reconstruction of V5 and V8 is performed.
Rationale of MRL graft and reconstruction of MHV tributaries
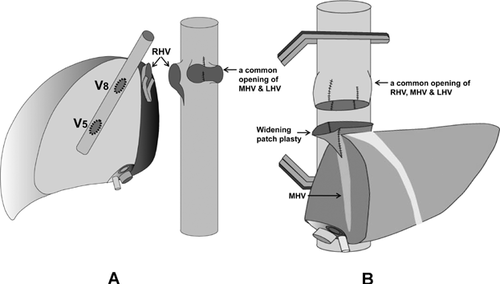
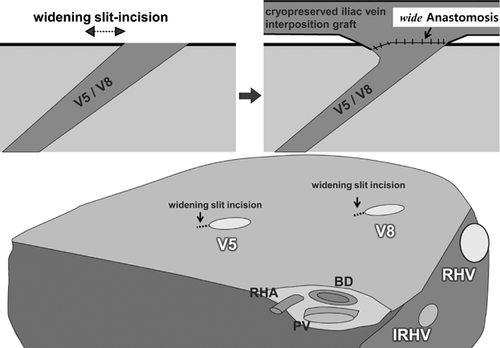
A right hemiliver graft with MHV reconstruction must be considered when the patient is seriously ill (MELD >20), graft size is relatively small (GRWR <1), the graft is obtained from an older donor (>50 years), the MHV is dominant over the RHV on donor's 3D CT or magnetic resonance, and the AS is larger than PS by volumetry CT. If preoperative 3D CT demonstrates sizable V5 and V8 (caliber >5 mm), MHV tributary reconstruction should be planned. Intraoperative estimation of congested volume of the AS is simply and accurately made during donor hepatectomy after complete transection of liver parenchyma. Discoloration of the liver surface of the AS will occur after 5 min of hepatic artery clamping. If the nondiscolored area is estimated at <50% of the recipient GV/SLV, MHV tributaries should be reconstructed 18. A previous report revealed that venous flow of ligated MHV tributaries into the RHV by way of intrahepatic collaterals might develop as early as 1 week after transplantation. However, the incidence of intrahepatic venous collateral formation between the RHV and MHV is estimated to be only 24%. Even though such collaterals are present, they are small and do not form a large trunk immediately after occlusion of the MHV. Congestion of the AS in an RL graft without MHV occurs in 85–88% of patients to varying degrees. Thus, our routine practice is the restoration of continuity of a sizable V5/V8 even if GRWR exceeds 1 (Table 1). Furthermore, even when the GRWR is <1 and a patient's preoperative status is poor, we attempt to reconstruct all small (down to 3 mm) MHV tributaries to maximize the functioning liver cell mass, or to use an ERL graft when the congestion-free remnant LL of the donor is more than 30% of TLV. Meticulous and aggressive reconstruction of MHV tributaries is vital for successful outcomes of the MRL graft. Early stenosis or technical occlusion of the anastomotic site between V5/V8 and the interposition vascular graft is higher when the orifice of V5/V8 is smaller than 5 mm or the interposition graft caliber is smaller than 10 mm. To alleviate anastomotic stenosis of V5/V8, the slit-incision of the orifices is widened in the caudal direction at the back table, and this simple procedure enlarges the orifice circumference nearly threefold (Figure 4). As an interposition vascular graft, autogenous GSV, PV bifurcation Y-graft, dilated umbilical collateral vein and HV excavated from resected liver have been used 19. Generally, a cryopreserved deceased donor iliac vein is the best conduit because of its adequate diameter (>10 mm), simplicity and excellent early patency rate (>90%). When cryopreserved homologous vessel grafts are not available, a synthetic vascular graft such as a ringed PTFE is an alternative with acceptable patency rate. Because of previously reported low patency rate of expanded PTFE grafts (i.e. 1- and 4-month patency rates of 80.8% and 38.5%) 20, detailed surgical techniques were devised and refined. We have used only ringed PTFE grafts of larger caliber (internal diameter >10–12 mm) combined with placement of composite vessel patches (autogenous GSV, cryopreserved artery from tissue bank) between V5/V8 and the PTFE graft 21. PTFE grafts without external rings are vulnerable to buckling and even shrinkage by external compression of a distended stomach, perigraft hematoma or fluid collection, but ringed PTFE grafts appear more resistant to those environments. Placement of composite vessel patches offsets stenosis-inducing tissue reaction of PTFE grafts to the direct anastomotic site of V5/V8, avoids tearing the thin-walled V5/V8 during suture, yields large orifices and allows redundancy without subsequent buckling of V8 anastomosis, forming a naturally coursing conduit appearance (Figure 5).
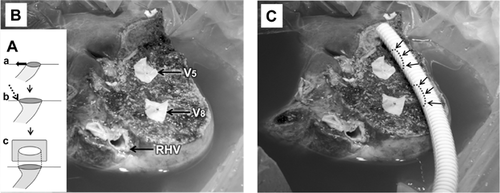
Expected short- and mid-term patency rates of cryopreserved iliac vein allografts and ringed PTFE grafts are 90% at 1 month and >60% at 6 months. The diameter of interposition grafts should be larger than 10 mm for prolonged patency. Currently, the proportion of patients requiring graft material for the MHV reconstruction in cryopreserved iliac vein, cryopreserved iliac artery, cryopreserved aorta and ringed PTFE is 46.6%, 16.4%, 5.0% and 32.1%, respectively, and the 6-month patency rate is 76.6%, 35.2%, 92.3% and 63.2%, respectively. Low patency rates of cryopreserved iliac arteries is explained by a small inner diameter (<7 mm) and frequently coexisting atherosclerosis. Unwillingness to use PTFE grafts in contaminated environments (i.e. bile leak) may rise from its increased vulnerability to bacterial colonization. To date, however, none of our patients with PTFE grafts (n > 350) have experienced significant infection. Antiplatelet therapy with aspirin is maintained for 6 months for PTFE graft patency.
To decrease V5/V8 reconstructions, adjacent tributaries are united into a single common opening with an intervening GSV or a cryopreserved arterial patch between them at the back table. In regards to selection of interposition graft material, a cryopreserved iliac vein graft is most suitable for MHV reconstruction, with less effort for bench work. PTFE grafts have the advantage of unlimited supply, and they appear more promising than previously recognized. A composite graft requires longer bench work and a small vessel patch. More than 60 min bench work does not provoke warm ischemic injury to liver graft. Long and concentrated bench work is rewarded by good patency of MHV reconstruction and saving patients' lives.
Reconstruction methods of ERL graft
Long-term patency of venous conduits draining AS in MRL graft is variable according to technique, number and size of V5/V8 and types of interposition vascular grafts. Along the course, the MHV may receive 15 tributaries from segment 5, and 18 tributaries from segment 8. Therefore, although the extent of the donor operation is increased, an ERL graft is generally more beneficial in regards to venous drainage than an MRL graft.
ERL grafts currently constitute only 5.7% (31/545) of our RL LDLTs (Table 2). Their mean GRWR (0.92) is smaller than those of RLs without MHV reconstruction (1.26%) and MRL grafts (1.15%) because we confine ERL LDLT to patients with small GRWR and high MELD scores, anticipating the perceived increased potential of donor morbidity and mortality risk in the absence of an MHV in the remnant left liver.
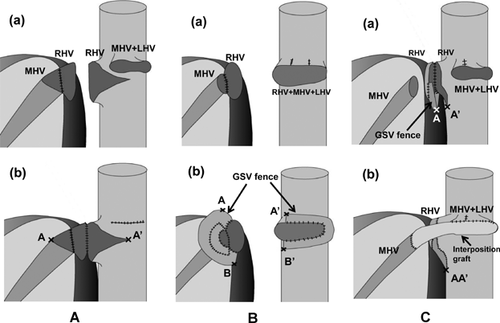
Various methods of HV reconstruction have been introduced to ensure good outflow drainage of ERL grafts. They are classified as follows: (1) short, direct anastomosis by unification venoplasty of the graft's RHV and MHV trunks, and corresponding triangular excision of the recipient's IVC 22 (Figure 6A); (2) redundant large dome-shaped reservoir anastomosis 23, 24 (Figure 6B); and (3) direct RHV anastomosis and separate reconstruction of MHV with an interposition graft (Figure 6C). Progressive graft regeneration may shift or rotate the dissection plane of the liver graft toward ventral and left sides in the small right subphrenic cavity. Consequently, this might make the MHV and its anastomosis vulnerable to distortion. Compared to the short, direct anastomosis, ventral interposition of the large redundant venous patch around the graft's RHV and MHV orifices is beneficial to offset this regeneration-induced distortion. A new dome-shaped vein cuff attached to the RHV and MHV orifices by quilt venoplasty with autogenous GSV is anastomosed to a common orifice of the recipient's RHV, MHV and left hepatic vein (LHV) (Figure 6B). Matching this superabundant dome-shaped ventral vein cuff to the enlarged HV orifice might permanently protect the HV anastomosis from stretching or compression, which otherwise could cause outflow obstruction 24. However, some drawbacks remain in this method for its complexity and the long wound at the recipient's groin GSV recover site. Despite its excellent patency, we switched our reconstruction method to separate anastomoses with the modification that an additional large-caliber vein conduit (cryopreserved common iliac vein, autogenous PV Y-graft or spirally oversewn GSV tube graft) is interposed between the graft's MHV and the common opening of the recipient's MHV and LHV (Figure 6C). Placement of large-caliber interposition conduits between both sides of the MHVs prevents stretching of the anastomotic site and allows redundancy that helps lessen compression of the anastomotic site by subsequent liver graft regeneration. This procedure is simple and does not need venovenous bypass.
Refinement of RHV and SRHV reconstruction
RHV reconstruction
The RHV is the primary outflow pathway for RL graft, and its successful reconstruction is essential for full graft function. An RL graft is approximately half the size of an adult standard liver volume. Thus, the graft rapidly regenerates in all directions within 2–3 weeks in the limited right subphrenic space, and this may compress the RHV anastomotic site 25. To cope with this conformational change, a wide ostium is indispensable for prevention of venous outflow obstruction. The majority of RHV reconstruction procedures incorporate enlargement of recipient's RHV or IVC and anastomotic orifice of the graft's RHV.
By CT follow-up routinely performed weekly during admission, 1 month and 1 year posttransplant, the most common form of RHV stenosis is anastomotic beaking at the caudal end of the anastomosis, and the longitudinal diameter of the RHV anastomosis decreasing at an average of 3.4 ± 1.2 mm at its root 26, 27.
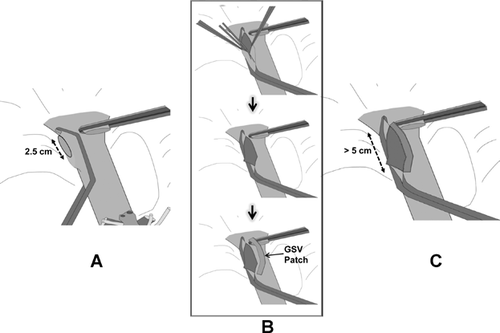
Other conformational changes by graft regeneration are tortuous stenosis of the hepatic IVC with oblique elliptical deformity, as well as marked stenosis of the confluence of the RHV across the anastomotic site by differing extents of liver regeneration in the anterior and posterior sectors by the failed AS drainage, for example, hypertrophy of the PS and atrophy of the AS. To obliterate anastomotic beaking caused by the acute angle between the graft RHV and recipient IVC, a downward incision is made at the caudal end of the graft RHV (Figure 7A and B). A near half-circumferential GSV patch plasty around the downward incision (Figure 7B and C) facilitates a wide, redundant anastomosis, hence avoiding IVC stenosis by corresponding enlarging IVC diameter.
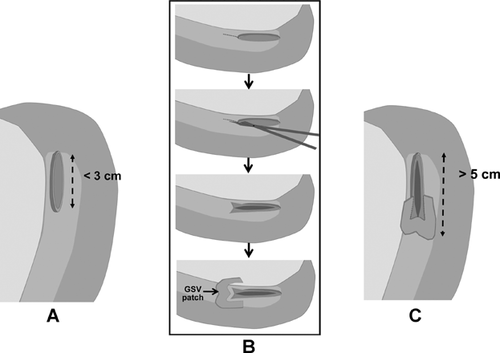
To counteract the conformational change of IVC constriction at the anastomotic site by regenerating graft compression, provision of a sufficiently sized dome-shaped reservoir on the ventral wall of anastomosis by aid of autogenous vein graft (GSV, PV) patch likely contributes to reduction of RHV anastomotic stenosis (Figure 8).
With the refined procedure of acute caudal angle blunting of the graft's RHV and IVC enlargement, the previous 4% incidence of RHV stenting for early RHV stenosis is decreased to 0.4% in our recent series 27. Also, MHV tributary reconstruction should be considered with RHV stenosis. In order to prevent uneven liver regeneration between the AS and PS that may contribute to morphological change of RHV anastomosis, MHV tributaries should be reconstructed whenever indicated.
SRHV reconstruction
A major SRHV (inferior RHV, middle RHV) larger than 5 mm in caliber has been indicated for revascularization, and is found in 42.7% of RL LDLT in our series. As separate anastomoses of multiple SRHVs are vulnerable to obstruction or regeneration-related torsion, creation of a common large opening of SRHVs may be beneficial for simple and safe anastomosis of multiple nonaligned SRHVs. The Tokyo University's group achieved complication-free reconstruction of SRHVs by using cryopreserved IVC graft 28. As the barrel of the IVC graft is large enough to encompass multiple SRHVs, that group's double caval method seems to be feasible in every variant of SRHVs (Figure 9B-a). However, it is practically impossible to obtain cryopreserved IVC or large-caliber vein grafts where deceased donor organs are scarce or tissue banks are not equipped. Our quilt venoplasty using autogenous GSV patchwork with or without circumferential patch-fence was initiated by lack of available cryopreserved vein grafts 25, 29.
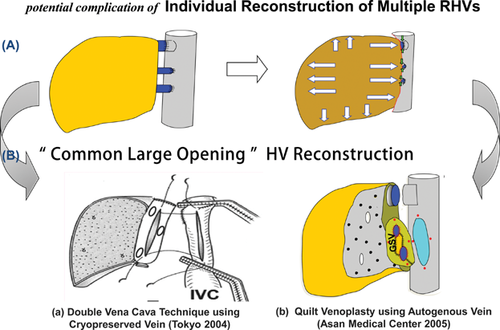
An alignment gap between SRHVs is simply offset by central placement of a rectangular GSV patch (Figure 9B-b). Quilt venoplasty using GSV offers several advantages over direct separate anastomosis or the double caval method. First, GSV is an autologous graft that can be applicable in all patients. Second, unlike direct separate anastomosis, GSV does not require a rim of IVC cuff around the SRHV of liver graft. This difference may secure donor safety by preventing potential risk of the IVC narrowing and subsequent thrombosis. Third, risk of anastomotic stenosis and torsion may be acceptably low with a wide, stable anastomosis. Prolongation of cold ischemic time at the back table to make a common opening is compensated for by shortening the graft implantation time.
Portal Hyperperfusion versus Portal Flow Steal
Virtually all living donor liver grafts in adult recipients are relatively SFS and require optimal portal inflow for immediate graft regeneration. Portal hyperperfusion is believed to deliver shear stress injury to hepatocytes of an SFS graft, which is already small to meet the metabolic demand of recipient. Various remedial procedures such as splenic artery ligation, splenectomy and small caliber hemi-portocaval shunt (HPCS) creation have been utilized to modulate portal inflow 12. However, high portal pressure is not related to portal blood flow alone, and liver graft outflow resistance also contributes. The safest, most effective way to manage portal hyperperfusion in SFS grafts is provision of good HV outflow (Figure 3) and modulation of high portal venous flow (PVF) and pressure (PVP) by splenic artery ligation. We do not perform splenectomy to decrease portal hyperperfusion because of its inherent complications such as bleeding, pancreatic fistula and abscess formation, and in cirrhotic patients, PV thrombosis. HPCS may provoke encephalopathy and graft atrophy by diversion and subsequent hypoperfusion of hepatotrophic portal inflow. In extreme instances, catastrophic consequences with allograft necrosis may occur with portal flow steal; thus permanent HPCS is not an appropriate choice for resolving portal hyperperfusion. PVF higher than 250 mL/min/100 gm graft weight or early elevation of PVP ≥20 mmHg after reperfusion is not associated with poor outcomes in SFS grafts as long as perfect venous outflow is provided and portal flow steal is completely interrupted. We discontinued measurement of PVP and PVF after introduction of IOCP and MHV reconstruction.
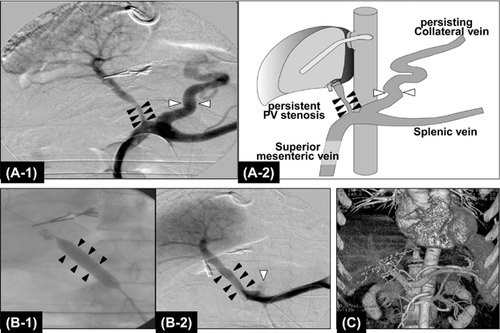
The lowest limit of graft size beyond which repair and regeneration are impossible is not well defined. Our lowest successful graft size in a portal hypertensive patient had a GRWR = 0.49, accompanied with PV stenosis and large spontaneous spleno-renal shunting collaterals (>2 cm). Uneventful recovery was seen with ligation of the left renal vein to interrupt large spontaneous spleno-renal shunt collaterals and correction of PV stenosis with augmentation patch venoplasty 30. In ALDLT, optimal portal inflow is a prerequisite for regeneration to meet the patient's metabolic demands. However, the decreased vascular bed of the partial liver graft will further enhance portal hypertension by increasing intrahepatic vascular resistance; the reduced vascular compliance plays a role in keeping porto-systemic (P-S) collaterals open. Portal flow steal through persistent collaterals often occurs early after LDLT and may result in hypoperfusion-induced graft necrosis and failure around 10 days postoperation. Since 2002, we have routinely performed interruption of large P-S shunting collaterals even in very SFS grafts (GRWR <0.6) after experiencing mortality related to devastating portal flow steal syndrome 31. Thirty percent of our cirrhotic patients who underwent LDLT demonstrated large (≥10 mm size) spontaneous P-S collaterals on preoperative 3D CT and underwent surgical ligation or coil embolization of collaterals via IOCP. Intraoperative doppler ultrasound (IOUS) has been used to ensure hemodynamics of the implanted liver graft. IOUS, however, has difficulty in evaluating correct anatomical and hemodynamic parameters of P-S collaterals. Even when IOUS showed adequate portal inflow during LDLT, portal flow steal syndrome manifested a few days after LDLT. To overcome the limitation of IOUS, IOCP has been used to evaluate portal flow to liver graft and to detect stealing hepatofugal flow through persistent P-S collaterals 32 (Figure 10). In addition, IOCP is therapeutically utilized to complete interruption of surgically inaccessible P-S collaterals by coil embolization and to treat PV stenosis interfering hepatopetal flow by stent placement 33. Although controversy exists, few authors present evidence of negative effects of persist spontaneous P-S collaterals in LDLT with SFS grafts and recommended their closure 34, 35. Inappropriate PVF may significantly reduce hepatic function in SFS liver grafts. To properly manage the potential SFS graft syndrome that may develop, both modulation of graft hyperperfusion by excessive portal hypertension and abolishment of portal flow steal through spontaneous or surgically created P-S collaterals are equally important.
Biliary Stricture
Biliary stricture (BS) remains the most challenging aspect of RL LDLT. BS significantly affects recipient quality of life and is occasionally a cause of graft and patient loss. Risk factors include number and size of reconstructed ducts, ischemic damage to the stumps of graft and recipient BDs, previous biliary leakage and the method of biliary reconstruction 36. Hepaticojejunostomy (H-J) was once the standard biliary reconstruction method, but has given way to duct-to-duct (D-D) anastomosis for its several advantages over H-J; not only is it faster and simpler to perform, but it preserves physiologic bilioenteric continuity, eliminates intestinal manipulation and the associated delayed return of bowel function, and facilitates endoscopic investigation and management of biliary complications 37. Regardless of technique used, a patient with multiple ductal openings has a higher incidence of BS than those with single duct. When an LL graft is used, BD reconstruction is generally straightforward because 88% of cases have single ductal orifices. In contrast, nearly 50% of RL grafts have two or three ductal orifices, and often two orifices are >1 cm apart.
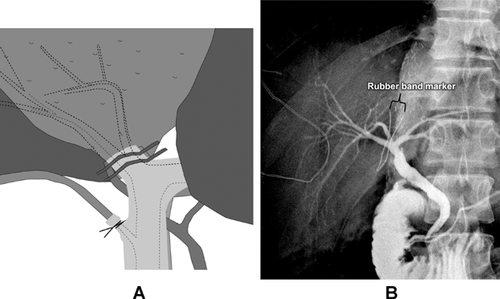
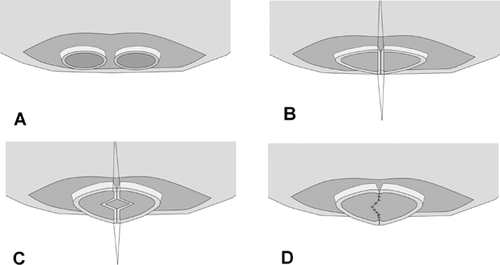
When RL grafts are to be used, precise investigation of the donor's biliary anomalies is of paramount importance to minimize the number of ductal reconstructions and to avoid injury to donor's BD near the hepatic duct confluence. To obtain coalesce BD openings, the right hepatic duct should be divided accurately by localizing the division site using a rubber-band tagging (RBT) method after near-complete parenchymal transection 38. To determine the BD division site, two short segments (10–15 mm in length, 1 mm in diameter) of the radiopaque rubber-band (taken from the blue radiopaque marker found in commercial surgical gauze) are tagged transversely 2 mm apart on the proposed division site of the right hepatic duct by fixing sutures to prevent displacement of the markers during manipulation of the liver (Figure 11). Intraoperative cholangiography through the cystic duct can identify the relation between RBT and the proposed duct division site. Once rubber-band tags are properly positioned at the planned division site, careful sharp division of the duct is made between the tags.
From November 2008 to December 2011, 1124 RL grafts at our center showed 570 single ductal orifices (50.7%), 200 single openings after ductoplasty (17.8%), 317 separate 2-BD openings (28.2%), thirty-five 3-BD openings (3.1%) and one 4-BD opening (0.1%). In the case of two adjacent ductal orifices, they are joined to form a common orifice for a D-D anastomosis. Ductoplasty is not suitable for ductal orifices that are further apart than the diameter of the larger ductal orifice. Inappropriate approximation of two ductal orifices under tension may cause ischemia, leakage and subsequent stricture of anastomosis. H-J is a better option in this situation. During ductoplasty, simply joining medial walls of two ducts will narrow the longitudinal diameter of a new opening and may further increase risk of BS. The newly created septum should be divided vertically and sutured transversely to transform a large opening (Figure 12). The net effect of septoplasty is a much larger orifice, which facilitates reconstruction and reduces BS formation 2. When two ductal orifices are far apart and use of a H-J is complicated by short and thickened mesentery such as is seen following peritonitis, modified techniques of D-D anastomosis using cystic ducts for reconstruction of one of the separated orifices 39, 40 or right and left hepatic ducts obtained by high hilar ductal division in the recipient are alternative options with acceptable complication rates. Modes of BD anastomosis in our experience has been D-D in 71.2%, H-J in 3.6%, double D-D in 17.6%, double H-J in 4.4% and combined D-D/H-J in 3.0%.
The role of stenting in biliary anastomosis creation is controversial. For a large ductal opening, a stent may not be necessary. For small openings, stents may help prevent occlusion of the anastomoses by edema early or prevent technical error such as catching of the posterior wall during placement of the anterior row of sutures. We routinely use small external drainage tubes exiting via the anterior wall of the common hepatic duct for several reasons. First, biliary drainage can prevent leakage by minimizing intraductal pressure at the anastomotic site. Second, external drainage tubes can help keep lumens open in early postoperative period; this may be important particularly when dealing with a very small (<2 mm) accessory duct or a spiral cystic duct. Third, it allows collection of information about bile production and hence about graft function. In addition, cholangiography can be performed to determine occurrence of leakage or stricture. In our RL LDLT, biliary leakage of D-D anastomosis has disappeared since routine application of small external drainage tubes (silastic straight-shape, 1.68 mm outer diameter, 0.76 mm inner diameter), together with preserving good arterial blood supply to the stumps of donor and recipient ducts by careful dissection. Overall the incidence of BS in our RL LDLTs has been <10% in single ductal opening, 14% in a ductoplasty opening, 24% in 2-ductal openings and 70% in 3-ductal openings. All strictures have been managed nonsurgically except 3 of our 3000 ALDLT patients, who ultimately required conversion from D-D to H-J. Two patients died of complications of endoscopic and percutaneous biliary intervention. Although patient and graft survival after ALDLT have approached those after DDLT, BS has been again identified as the Achilles' heel of this procedure, affecting approximately 20–25% of recipients. BS leading to patient death is negligible in our institute. Expert and dedicated interventional radiologists and endoscopists are absolute prerequisites for a successful LDLT program.
Current Strategies to Expand ALDLT With Broadened Donor and Patient Selection Criteria
Dual LDLT
One-third of potential live donors are not accepted as liver donors for adult recipients because of advanced age, steatosis, small RLV and calculations suggesting an SFS graft. To ensure donor safety and avoid SFS grafts, dual LL LDLT was introduced in 2000 7. When the available single RL graft cannot meet the recipient's metabolic demand, dual LDLT using RL and LL grafts can expand application of ALDLT by satisfying required GRWR of recipients 41. Mean GRWR with dual LL LDLT (median, 0.9; range, 0.59–1.2) approaches that of an RL LDLT (median, 1.15; range, 0.56–2.63). Prevalence and severity of donor complications of dual LL LDLT were lower than those of single RL LDLT. The most common complications in dual transplants were BS (21%) and unilateral HV obstruction of the heterotopic right-sided LL graft (15%). HV outflow obstruction is related to progressive compression of HV anastomosis by the regenerating liver graft. Frequently, outflow obstruction developed in the early posttransplant period (<3 weeks) and required ballooning and stenting to relieve congestion and restore normal liver function. Outflow obstruction rarely occurs in orthotopically positioned grafts.
From March 2000 to February 2011, 300 (13.5%) of our 2227 ALDLT were dual transplants using the same patient selection criteria as RL LDLT. Urgent dual transplants were performed for 18 FHF (6.0%) and 44 ACLF (14.7%) patients. The incidence of biopsy-proven AR was similar to that of single-graft LDLT recipients (17%); three-fourths of AR cases developed simultaneously in bilateral grafts. The in-hospital mortality rate was 7.0% (21/300), and high-urgency patients comprised 52% (11/21) of all mortality. In 8% of our dual transplants, unilateral graft atrophy insidiously developed over 1–2 years in one of two LL grafts by progressive outflow obstruction or portal flow steal between two grafts, but this did not affect liver function or survival. LDLT with dual LL grafts is technically complex and elaborate; it can help solve problems related to SFS graft and expand the donor pool. With application of dual transplant, ALDLT volume increased 13.5% at our institute.
Engraftment procedures using two LL grafts were previously described 7.
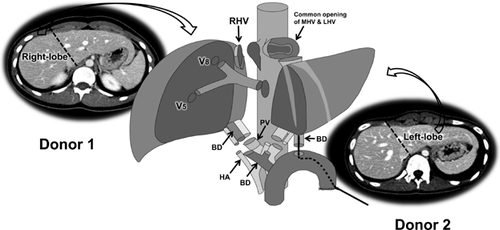
Engraftment procedures using both RL and LL grafts
Before implantation of the liver grafts, venoplasty of the HVs in the recipient and the grafts should be performed to make wide outflow orifices to prevent outflow stenosis. The recipient's RHV should be enlarged and elongated by longitudinal incision of the IVC at the inferior corner and fencing with the bisected autogenous GSV. The MHV and LHV are also converted into a single opening by division of the septum, and are enlarged and elongated by adding a transverse incision at the right corner and fencing with the bisected GSV for a wide, redundant HV anastomosis. At the back table, venoplasty to widen the HVs of two liver grafts is routinely needed for size-match with the enlarged recipient HVs. If autogenous GSV remains, fencing of the left-positioned LL graft allows for a good operative view during anastomosis of the redundantly lengthened LHV without tearing the vein wall, and might help avoid postoperative outflow stenosis. Venous obstruction is more common following an anastomosis that is perceived as difficult to complete.
Implantation proceeds in the following order. First, the RL graft is placed in the right upper quadrant and reconstruction of the RHV and short RHVs, if they exist, is performed. Reconstructed MHV tributaries of MRL graft are anastomosed to the anterior wall of the vena cava 2–3 cm below the recipient's LHV and MHV common orifice. If this is too low, the right PV may be compressed by the reconstructed MHV interposition conduit. Second, the LL graft is placed orthotopically and its HV and PV are reconstructed sequentially. Third, the anastomosis between the recipient's right PV and RL graft's PV is performed, and then both liver grafts are reperfused simultaneously. Last, after completion of the hepatic artery anastomoses, biliary reconstruction to both grafts is performed using a Roux-en-Y H-J or combination of D-D and H-J to each graft (Figure 13).
LDLT Across ABO-Blood Group Incompatibility
Paired donor exchange program
To cope with ABO-incompatibility and to avoid the potential complication of ABO-i ALDLT, a donor exchange program for ALDLT was initiated in 2003 at our institute 42. Our donor exchange program for ALDLT differs from normal ALDLT in three aspects. First, the donor exchange uncouples the typical connection between live donors and recipients. Second, donation through the exchange donor program is legally regarded as an unrelated donation in Korea. Thus, approval by the Ministry of Health and Welfare Korean Network for Organ Sharing requires more strict qualifications than for the usual related donations. Third, for exchange ALDLT, two sets of LDLT operations should be performed simultaneously to prevent potential conflicts from different outcomes between pairs. This requires four separate surgical teams and facilities; hence, exchange LDLT may be feasible only in a few high-volume LDLT centers. For exchange LDLT, the principle of equality should be emphasized so that paired recipients are not disadvantaged by unfavorable outcomes.
Conditions between donors and recipients must be matched to minimize donor morbidity and to maintain an acceptable recipient survival rate. Consequently, we tend to select patients with relatively low MELD scores and donors showing RL volume equivalent to an estimated GRWR >0.8. Between July 2003 and December 2012, 13 pairs of donor exchange ALDLTs were performed with two pairs of high-urgency patients, and 1 recipient of the pairs underwent two LL dual transplants because two potential donors directed to him demonstrated a disproportionately small LL (<30% TLV). All donors recovered uneventfully. Twenty-five of 26 recipients survived, but one died of pneumonia 54 days posttransplant. Despite vigorous efforts to increase paired exchange ALDLT, only one exchange pair per year has been successfully established among a pool of 20 ABO-mismatched pair candidates; this constitutes less than 1% of our annual ALDLT. It seems that liver transplant candidates are more concerned about ABO-i LDLT than the paired donor exchange program because donor exchange uncouples the emotional relationship between the donor and recipient, even though they are informed about the potential disadvantage of ABO-i LDLT such as antibody-mediated rejection (AMR), diffuse intrahepatic biliary stricture (DIHBS) and long hospital stays for preoperative desensitization. Although only 13 pairs of ALDLTs were performed, it seems to be a feasible modality to overcome ABO-incompatibility, even in high-urgency patients.
ABO-i ALDLT
Aside from donor safety and graft-to-recipient size match, ABO-compatibility has been regarded as an essential prerequisite for successful LDLT. We initially hesitated to perform ABO-i ALDLT due to concerns for early graft loss, although the outcome of our consecutive cases in pediatric patients was successful. However, the outcomes of ABO-i ALDLT have remarkably improved since 2007 due to a set of innovative perioperative management tools to overcome ABO-blood group barrier 43. We launched the ABO-i ALDLT program in 2008, encouraged by noticeable success of this procedure in Japan and by a rapid increase of our DDLT numbers that could make DDLT allowable for high-urgency patients who would fail ABO-i LDLT by AMR.
Although there are practice differences among centers, preoperative management for ABO-i recipients has included of plasmapheresis (PE), rituximab and postoperative local infusion therapy (LIT) of immunosuppressants via the PV or hepatic artery, with or without splenectomy. Preoperative PE is an effective modality to decrease the titer of preformed antibody, aiming for preoperative titer of less than 1:8. It is also repeated as a rescue therapy during postoperative AMR or when elevated antibody titers are seen. With LIT, the incidence of catheter-related complications was fairly high (22.4%) and often life-threatening; they include hepatic artery thrombosis (HAT), bleeding with catheter dislodgement and catheter insertion site infection 44. Serious catheter-related complications in the first 20 ABO-i cases, prompted us to stop LIT, and adopt a new protocol of rituximab, PE, intravenous immunoglobulin and triple immunosuppressive therapy (tacrolimus + mycophenolate mofetil + steroids). From November 2008 to September 2012, 161 (13.1%) ABO-i LDLTs were performed. Currently, ABO-i ALDLT comprises up to 25% of all ALDLTs with comparable results to ABO-c ALDLT. There have been no episodes of AMR in the last 161 recipients, and 3-month, 1-, 2- and 3-year graft survival rates have been 98.7%, 95.8%, 91.8% and 91.8%, respectively. Nine patients (5.6%) were complicated by DIHBS which occurred 2.1–5.2 months posttransplant. The most important factor affecting recipient survival was preoperative disease severity. Subjects with high MELD score and poor performance status were usually vulnerable to infectious complications, that was potentially accelerated by B cell depleting agents and more potent immunosuppressive regimens. We currently confine ABO-i ALDLT to patients with a MELD <30, excluding high-urgency patients. ABO-i ALDLT can be a feasible option for end-stage liver disease in countries with a shortage of deceased donors when there is no available ABO-c live donor.
LDLT for Unresectable HCC in Cirrhotic Patients
Over the last decade, liver transplantation in Asia has increased 10-fold, largely as a result of development of LDLT and high incidence of HCC. Presently, HCC comprises half the indications for ALDLT in Asia.
There are two controversial issues concerning LDLT for HCC. First, LDLT has a higher recurrence rate than DDLT even in HCC patients within Milan criteria. Possible explanations for the difference include the ability to proceed with LDLT early in the course of the disease, thus eliminating natural selection of aggressive tumors that occurs during prolonged waiting period, and SFS graft regeneration that may biologically enhance tumor growth and invasiveness. Moreover, the piggyback vena cava dissection in the recipient is a less-than-optimal cancer operation with more manipulation of the tumor leading to tumor embolization through the HV and increased potential for leaving residual tumor cells. A GRWR of <0.8 was not a significant risk factor of HCC recurrence in our previous study 45. To minimize manipulation of the tumor and reduce early recurrence of HCC in LDLT, we have, since 2009, performed 13 en bloc total hepatectomies and IVC replacements with artificial interposition vascular grafts for HCC located in paracaval portion with encouraging results 46.
Second, critics question if expanded selection criteria for LDLT are justified for patients with advanced HCC. Milan criteria still remains the gold standard, with 4-year patient survival rates of 75% 47. But many HCC patients who would be excluded because they do not meet Milan criteria could obtain survival benefit relative to nontransplant options. With LDLT, liver grafts are considered as private gifts instead of a public resource. Modest expanded criteria for patient selection are, we feel, acceptable and necessary in LDLT 48, 49. We have observed no definite difference in recurrence rates for patients with up to six tumor foci so long as the largest tumor diameter does not exceed 5 cm. To improve results of LDLT for advanced HCC with expanded criteria, downstaging to within-Milan criteria is intentionally advocated using locoregional therapy with transcatheter arterial chemoembolization, radiofrequency ablation, ethanol injection and radiation. Advanced HCC that shows good response to downstaging therapy has a tendency to obtain a satisfactory long-term survival after LDLT.
Salvage LDLT is often feasible in previously hepatectomized HCC patients because many intrahepatic recurrences frequently remain transplantable 50. Basically, prognostic selection criteria for salvage LDLT are the same as for primary LDLT. But when the histology of the previously resected HCC demonstrates poor prognostic signs for early recurrence, such as vascular invasion, poor differentiation, combined HCC with cholangiocellular carcinoma, and satellite nodules, salvage LDLT should be cautiously considered even in patients with HCC within-Milan criteria. Among 2778 ALDLTs, 100 salvage LDLT (8.7% of ALDLT for HCC) for recurred HCC with or without hepatic decompensation were performed with a 5.1% in-hospital mortality, 2.5 times higher that of primary LDLT. Operative mortality was higher in portal hypertensive patients who underwent previous major hepatectomy 50. The current 76.9% of overall 5-year survival rate of salvage LDLT for HCC patients within-Milan or modest expanded criteria is comparable to that of primary LDLT.
LDLT for High-Urgency Patients
Although LDLT has allowed optimal timing of surgery for FHF and ACLF, results of urgent LDLT are acknowledged to be inferior to those of elective LDLT; this is largely due to patient comorbidities and SFS graft. It is still debated whether decompensated patients can tolerate RL LDLT. The reported mortality rate following LDLT (57%) versus DDLT (18%) was threefold higher for patients with ACLF 51. To achieve results comparable to DDLT, size-matching to a GRWR of 1.0 or a 50% GV/SLV with liver grafts functioning immediately is required; technical complications are not tolerated in urgent LDLT. A fully functioning liver graft is ensured with no graft congestion by perfect HV outflow reconstruction, use of a young, nonsteatotic donor and no persistent portal flow steal. This latter point can be facilitated by interruption of sizable P-S collaterals. An RL graft with MHV reconstruction is a durable graft even for patients with very high metabolic demands 5. In order for the already desperately ill patients not to further deteriorate, there should be no bleeding or septic complications such as bile leak, hemobilia or early BS. In addition, early enteral nutrition through a feeding jejunostomy is routinely applied to decrease bacterial infections. An SFS graft predisposes a patient to increased PVP and splanchnic congestion, impaired bowel motility, bacterial translocation and sepsis. Enteral nutrition stimulates bile flow and portal blood flow, prevents intestinal mucosal atrophy, preserves intestinal smooth muscle structure and function and, finally, contributes to a decrease in septic complications. Before application of urgent LDLT, fewer than 5% of these critically ill patients benefited from DDLT due to deceased donor graft shortage in our region. But LDLT has allowed 75% of our high-urgent patients to undergo transplant with 1-year survival rate of 85% 5, 51, 52.
LDLT for Challenging Cases
Re-LDLT
The reported rate of retransplants from large-volume DDLT programs in Western countries has varied from 7% to 23%. In contrast, of the first 1000 ALDLTs at our institute, 1.9% underwent living donor retransplant 19. We attribute our low rate to several reasons.
First, our incidence of primary nonfunction (PNF) was very low after LDLT. The US A2ALL study reported that 11 (2.9%) LDLT failed because of PNF in 385 recipients 53. In contrast, our incidence of PNF after ALDLT is 0.1%. Severe initial dysfunction of liver grafts have often been associated with SFS graft, excessive congestion of RL grafts from outflow obstruction, or portal flow steal syndrome. Hepatic artery-related complications, HAT or pseudoaneurysm occurred in 2–5% of recipients at high-volume LDLT programs and became a leading cause of early graft failure. Intractable biliary complications can also be an indication for retransplant, especially in DIHBS after ABO-i LDLT. Second, advancement of surgical, radiologic and endoscopic interventional technique has contributes to our lower re-LDLT rate. Third, many families lacked a next-available living donor for re-LDLT. In Asian countries where deceased donor grafts are scarce, the probability of retransplant is very low. Thus, serious posttransplant complications often lead to death with no chance of retransplant. The 1-year patient survival rate of re-LDLT was 60%, much lower than the 94% rate of primary LDLT in adult patients. The most important concern in technical feasibility of re-LDLT is availability of hepatic arterial inflow source. Reuse of the proper hepatic artery branch in recipients with HAT is not possible in most instances. As an alternative, autogenous inferior mesenteric or sigmoidal artery interposition grafts can be used, but the available length of artery may be insufficient.
A reliable source of hepatic artery inflow is recipient's right gastroepiploic artery because of its invariable location, as well as its sufficient length and size. The right gastroepiploic artery often appears too small, but it can be rapidly dilated after clamping for a short time with concomitant splenic artery ligation. If the PV is injured or surrounded by dense adhesion during dissection so that an adequate length of recipient PV cannot be secured for tension-free PV anastomosis, a tube interposition graft made from autogenous GSV is the best substitute to complete portal continuity in our practice.
PV reconstruction with ringed PTFE graft
In patients with complete obliteration of the PV with diversion of the portal flow through spontaneous large reno-portal or other P-S collaterals, PV reconstruction with a ringed PTFE vascular graft for reno-portal or mesenterico-portal anastomosis allows timely LDLT in the absence of a fresh deceased donor interposition vascular graft with good outcomes. We have successfully performed a PTFE jump graft in nine reno-portal and three mesenterico-portal anastomoses 54.
Atrio-caval anastomosis for Budd–Chiari syndrome
HV occlusion in patients with Budd–Chiari syndrome presents particular technical challenges. Depending on the location of the thrombotic occlusion, different approaches such as cavoplasty and IVC replacement with deceased donor IVC might be necessary. At our institution, IVC replacement with a large-caliber artificial interposition vascular graft (>35 mm Dacron tube) between the right atrium and the infrahepatic IVC was first introduced in 2006. Cavoplasty only is not practical because dissection of the retrohepatic IVC results in torrential bleeding, and the paper-thin IVC wall is prone to be torn and unusable with venoplasty. All six patients who underwent IVC replacement between the right atrium and the infrahepatic IVC have survived without IVC stenosis or thrombotic complications.
Important Aspects of Teamwork for Success
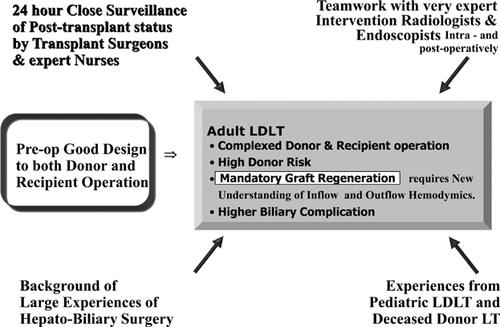
ALDLT is a life-saving procedure, but it is often performed under less-than-ideal conditions. The safety margin is small for both recipients and donors. Every step of the procedure must be planned and performed meticulously and tailored to each individual. Postoperative intensive care, including ECMO support for acute pulmonary failure and continuous renal replacement therapy for hepato-renal syndrome, is often required.
The possibility of developing surgical complications is higher in this challenging procedure compared to DDLT. A multidisciplinary approach with surgical, radiological and medical teams, and a wide range of ancillary services, which can be provided with institutional support, is crucial (Figure 14). True institutional support requires a willingness to invest in LDLT for the establishment of a dedicated staff for these resource-intensive procedures, provision of adequate operating room and intensive care unit services, specialized instruments, and experienced personnel. All this is required despite the potential negative income balance compared to other surgical procedures. ALDLT programs are best reserved for institutions capable of delivering the required experienced surgeons and team-oriented interventionists along with all of the necessary institutional resources, in the context of a waitlist mortality for DDLT that is >10% despite all efforts to increase the deceased donor pool 55.
Conclusion
Efforts to match the supply of deceased donors for all patients requiring liver transplantation have been limited. Living donors represent a large pool of organs and seem to be the only logistically practical and immediately available alternative. However, ALDLT is a technically challenging procedure immersed in inherently complex ethical issues. In these interventions, donor safety constitutes the main priority, and recipient safety with preservation of valuable liver grafts is next in importance. As in other demanding procedures, the development and results of ALDLT are strongly tied to the experience and commitment of the surgical team. Over the past decade, most of the issues related to the technical design of ALDLT procedures have been solved. For optimal performance of LDLT in adult patients, a comprehensive understanding of the dynamic nature of regenerating partial liver grafts is required, and applied to overcome HV outflow insufficiency and portal inflow steal. Based on our experiences, techniques described herein have demonstrably diminished the morbidity and mortality associated with technical errors during ALDLT procedures.
Acknowledgments
I sincerely thank all members of our liver transplantation program for their enormous dedication and cooperation in performing more than 400 liver transplants per year: C.S. Ahn, D.B. Moon, T.Y. Ha, G.W. Song, S. Hwang, K.H. Kim, D.H. Jung, G.C. Park and J.M. NamGoong of hepato-biliary surgery and liver transplantation; G.B. Sung and G.Y. Ko of interventional radiology; S.K. Lee of pancreato-biliary endoscopy; G.S. Hwang, Y.K. Kim and S.M. Jueng of anesthesiology; H.W. Lee in surgical suite; S.R. Kim and S.H. Lee in surgical ICU; H.S. Ha in Organ Transplantation Center.
Disclosure
The author of this manuscript has no conflicts of interest to disclose as described by the American Journal of Transplantation.