Emerging Role of Gasotransmitters in Renal Transplantation
Abstract
Once patients with kidney disease progress to end-stage renal failure, transplantation is the preferred option of treatment resulting in improved quality of life and reduced mortality compared to dialysis. Although 1-year survival has improved considerably, graft and patient survival in the long term have not been concurrent, and therefore new tools to improve long-term graft and patient survival are warranted. Over the past decades, the gasotransmitters nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) have emerged as potent cytoprotective mediators in various diseases. All three gasotransmitters are endogenously produced messenger molecules that possess vasodilatory, anti-apoptotic, anti-inflammatory and anti-oxidant properties by influencing an array of intracellular signaling processes. Although many regulatory functions of gasotransmitters have overlapping actions, differences have also been reported. In addition, crosstalk between NO, CO and H2S results in synergistic regulatory effects. Endogenous and exogenous manipulation of gasotransmitter levels modulates several processes involved in renal transplantation. This review focuses on mechanisms of gas-mediated cytoprotection and complex interactions between gasotransmitters in renal transplantation.
Abbreviations
-
- 3MP
-
- 3-mercaptopyruvate
-
- 3MST
-
- 3-mercaptopyruvate sulfurtransferase
-
- BAI
-
- 7-butylhexahydro-1H-azepin-2-imine
-
- BD
-
- brain dead
-
- BR
-
- biliverdin reductase
-
- CAT
-
- cysteine aminotransferase
-
- CBS
-
- cystathionine β-synthase
-
- (c)GMP
-
- (cyclic) guanosine monophosphate
-
- CO
-
- carbon monoxide
-
- COHb
-
- carboxyhemoglobin
-
- CoPP
-
- cobalt protoporphyrin
-
- CORMs
-
- CO-releasing molecules
-
- CRTF
-
- chronic renal transplant failure
-
- CSE
-
- cystathionine γ-lyase
-
- DAO
-
- D-amino acid oxidase
-
- DCD
-
- donation after cardiac death
-
- DGF
-
- delayed graft function
-
- eNOS
-
- endothelial nitric oxide synthase
-
- ESRD
-
- end-stage renal disease
-
- GC
-
- guanylate cyclase
-
- GFR
-
- glomerular filtration rate
-
- GTP
-
- guanosine triphosphate
-
- H2S
-
- hydrogen sulfide
-
- HO-1, HO-2, HO-3
-
- heme oxygenase-1, -2 and -3
-
- iNOS
-
- inducible nitric oxide synthase
-
- IOPS
-
- isolated organ perfused system
-
- IRI
-
- ischemia-reperfusion injury
-
- KATP channels
-
- ATP-sensitive potassium channels
-
- L-NAME
-
- L-NG-nitroarginine methyl ester
-
- L-NIL
-
- iminoethyl-lysine
-
- MAPK
-
- mitogen-activated protein kinases
-
- NaHS
-
- sodium hydrosulfide
-
- Na2S
-
- sodium sulfide
-
- NF-κB
-
- nuclear factor kappa B
-
- NHB
-
- non-heart-beating
-
- nNOS
-
- neuronal nitric oxide synthase
-
- NO
-
- nitric oxide
-
- NOS
-
- nitric oxide synthases
-
- Nrf-2
-
- nuclear factor-like-2
-
- PDE-5
-
- phosphodiesterase-5
-
- PPG
-
- propargylglycine
-
- SNP
-
- sodium nitroprusside
-
- SnPP
-
- tin protoporphyrin
-
- VEGF
-
- vascular endothelial growth factor
Introduction
The incidence and prevalence of end-stage renal disease (ESRD) have increased steadily in recent decades. This undoubtedly reflects the aging population in combination with increasing rates of risk factors involved in the development of renal disease such as hypertension, diabetes mellitus type 2 and obesity. For the majority of patients with ESRD, renal transplantation is the preferred treatment option, since it ends the need for debilitating dialysis and markedly improves quality of life and life expectancy as compared to patients on dialysis. Unfortunately, even after successful renal transplantation, morbidity and mortality rates are notably higher compared to those in the general population. Short-term survival is not the main factor that constitutes a threat to renal transplant recipients, since 1-year survival has improved considerably with a 90% and 95% chance of a functioning graft for recipients of deceased and living kidney transplants, respectively. These encouraging numbers are attributed to improvement of surgical procedures, prevention of acute rejection episodes, better treatment of opportunistic infections and introduction of more effective immunosuppressive agents. However, graft and patient survival in the long term have not been concurrent. Of all patients surviving the first year after transplantation, 50% of the renal grafts originating from deceased donors are lost within 12 years after transplantation. One of the major causes of graft loss is the development of renal transplant failure, which is related to immediate and long-term effects of renal ischemia-reperfusion injury (IRI). Maintaining organ viability between donation and transplantation is of critical importance for optimal graft function and survival. New strategies that intervene in the stressful events during and after transplantation are needed. In the ceaseless search for tools to improve long-term graft and patient survival, gasotransmitters may provide us with novel and exciting therapeutic opportunities.
Origin of Gasotransmitters
The gasotransmitters nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) were abundantly present in the prebiotic earth. There is ample scientific evidence that they are involved in the origin of life and acted particularly creatively in the endosymbionic event that gave birth to the development of mitochondria. When the earth oxygen levels were rising, NO, CO and H2S “vaporized” and cells started to utilize metabolic pathways to compartmentalize and regulate these gases for signaling purposes. Gasotransmitters have evolved significantly in vertebrates and still participate in biological regulatory systems associated with cellular energy and oxygen delivery. Compelling evidence for this comes from data showing that H2S producing enzymes either are present within the mitochondria 1 or translocate toward mitochondria under hypoxic conditions 2. These gases, despite their volatile character, cannot escape detection because of the footprints they leave behind in tissues and body fluids. NO can be detected by its end-products nitrite and nitrate, CO by carboxyhemoglobin (COHb) and H2S by thiosulfate, sulfite, sulfate, sulfhemoglobin and sulfhydrated proteins.
(Patho-)Physiological Aspects of Gasotransmitters
Since its discovery as an endothelium-derived relaxing factor, NO has been the most studied gasotransmitter and its formation from the conversion of L-arginine to L-citrulline by nitric oxide synthases (NOS) has been well characterized (Figure 1). Three NOS subtypes can be distinguished; the first, that is inducible NOS (iNOS), is responsible for NO production in inflammatory conditions and immune responses. iNOS knockout mice are sensitive to infections but resistant to sepsis-induced hypotension. In the kidney, iNOS is predominantly found in infiltrating macrophages during inflammation (Figure 2). Endothelial NOS (eNOS) is constitutively expressed in endothelial cells (Figure 2) and closely involved in the regulation of vascular tone. Consequently, eNOS knockout mice develop hypertension and perturbed hemodynamic homeostasis 3. Neuronal NOS (nNOS), also a constitutively expressed form, is predominantly present in neuronal cells and the renal macula densa. In general, NO modulates platelet aggregation, leukocyte adhesion and smooth muscle cell relaxation. Intriguingly, nitrite, the “stable” end-product of NO, can be converted to NO during deoxygenation of hemoglobin in the microcirculation 4 or by eNOS 5. In the kidney, nitrite is reduced to NO by xanthine oxidase reductase, making dietary or supplementary nitrite an attractive therapeutic option. The reaction of NO with superoxide anion produces peroxynitrite (ONOO−), a potent oxidant and nitrating species that can cause depletion of the important anti-oxidant glutathione by oxidation of sulfhydryl groups.
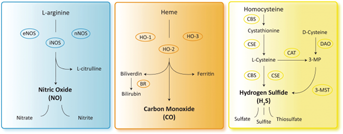
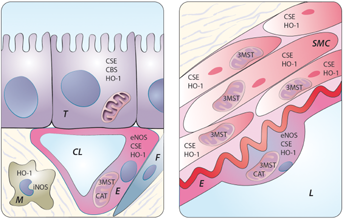
Using oxygen and reduced nicotamide adenine dinucleotide phosphate as a source of energy, CO can be synthesized during the conversion of heme to biliverdin by three enzymes: heme oxygenase-1, -2 and -3 (HO-1, HO-2 and HO-3) (Figure 1). HO-1 basal levels are highest in tissues that participate in erythrocyte degradation. CO is involved in various processes including the regulation of endothelial cell survival and proliferation, protection against IRI, vasorelaxation and inhibition of pro-inflammatory responses 6, 7. HO-1 is expressed in cortical tubules and the renal vasculature under physiologic conditions and is strongly induced under oxidative, chemical and physical stress (Figure 2). In disease states, HO-1 acts as a protective factor during stress-induced inflammatory injury. HO-1 knockout mice are sensitive to oxidative stress, indicating that the ability of an organism to up-regulate HO-1 may serve as an adaptive anti-oxidative mechanism 7. HO-2 is constitutively expressed in the brain, while its presence in human kidneys is unresolved. Although studies have indicated an important role for HO in protecting the renal vasculature from excessive vasoconstriction, this does not seem to be related to HO-2. HO-3 is an alternatively spliced version of HO-2 with lower enzymatic activity and its physiological significance is currently not well understood.
H2S is derived from L-cysteine via cytosolic enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) or from D-cysteine by the tandem enzymes cysteine aminotransferase (CAT) and 3-mercaptopyruvate sulfurtransferase (3MST) (Figure 1) 8. CBS and CSE are present in a variety of tissues 9 including kidneys 10, whereas 3MST expression has been mainly demonstrated in the vascular endothelium (Figure 2) 8. Human CBS deficiency leads to severe hyperhomocysteinemia with a high risk for developing vascular diseases; CSE deficiency leads to a rare disorder named cystathioninuria. Genetic knockout of CSE in mice leads to an age-dependent increase in blood pressure, indicating a critical functional role for CSE-derived H2S in the regulation of vascular tone 11. While homozygous CBS knockout mice die within days to weeks, heterozygous variants similarly develop an elevated blood pressure 12. H2S induces the relaxation of blood vessels by mechanisms that include the activation of ATP-sensitive potassium channels (KATP channels), possibly through direct cysteine-S-sulfhydration. H2S also has anti-inflammatory features, such as the inhibition of pro-inflammatory transcription factors like nuclear factor kappa B (NF-κB) and the inhibition of pro-inflammatory enzyme activity of iNOS, cyclooxygenase-2 and tumor necrosis factor-α converting enzyme 13. Furthermore, H2S promotes angiogenesis and anti-oxidant activity and has cytoprotective effects through anti-apoptotic and -necrotic mechanisms 10, 14, 15.
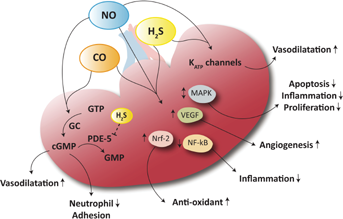
Interactions Between Gasotransmitters
Based on the resemblance between the signaling roles of NO, CO and H2S, it is plausible that interactions take place. Each gas may antagonize, reciprocally regulate or potentiate the cellular effects of each other through their production, downstream molecular targets and direct chemical interactions (Figure 3). Gasotransmitters have structural similarities to molecular oxygen and can bind heme groups. At a vascular level they share vasorelaxing properties via cyclic guanosine monophosphate (cGMP) and KATP channels. Furthermore, they affect several mutual intracellular pathways such as nuclear factor-like-2 (Nrf-2), NF-κB and several mitogen-activated protein kinases (MAPK), thereby exerting anti-oxidant, anti-inflammatory and anti-apoptotic effects (Figure 3). Additionally, they can suppress the increased expression of Toll-like receptors in response to endogenous damage-associated molecular pattern molecules 16, 17. NO induces the production of CO in vascular smooth muscle cells, and CO directly inhibits iNOS activity by binding to its heme moiety. By doing so, CO acts as a cellular protector by decreasing the amount of NO during, for example, oxidative stress 18. During hypoxia, the down-regulation of eNOS coincides with a transient increase in HO-1 protein, suggesting compensatory regulation. Similarly, H2S can inhibit iNOS directly 13 and up-regulate HO-1 expression in human podocytes and mesangial cells, thereby inhibiting inflammation 19. The vasorelaxant effects of H2S may be dependent on NO, since inhibition of NO using L-NG-nitroarginine methyl ester (L-NAME) decreases the potency of H2S in aortic rings 20, indicating that H2S stimulates NO production. However, through the possible formation of a nitrosothiol intermediate, the vasorelaxant effect of NO is inhibited by H2S, further suggesting reciprocal regulation of their biological activity in the vasculature. In addition, H2S prevents the degradation of cGMP by inhibition of phosphodiesterase-5 (PDE-5), thereby stabilizing the effects of NO. Furthermore, H2S is able to up-regulate eNOS, thereby potentiating NO production 21. Conversely, endogenous production of H2S can be stimulated by NO donors mediating a stimulatory effect of NO on CBS and/or CSE 22. Various interactions are likely to be discovered since it is known that the activity of human CBS may be regulated by heme-mediated redox-linked mechanisms.
Gasotransmitters in Renal Transplantation
NO in renal transplantation
Both protective and detrimental properties have been described for the role of NO in renal transplantation. During acute rejection and chronic renal transplant failure (CRTF) there is an increase in glomerular and interstitial iNOS expression together with a loss of glomerular eNOS expression. In CRTF, the increase of iNOS was concurrent with a significant increase in the production of reactive oxygen species and nitrotyrosine positive cells indicative of damage by peroxynitrite. The use of immunosuppressive drugs also directly affects NO production and NOS expression. Administration of an NO substrate (L-arginine) NO donors (e.g. molsidomine), and transfer of the NOS gene to the endothelium, are strategies to restore NO availability. In experimental studies L-arginine improved renal graft function and survival in the acute phase of renal transplantation, and attenuated proteinuria and glomerulosclerosis in CRTF. In clinical studies, L-arginine only improved renal hemodynamics in renal transplant patients not receiving cyclosporine 23. L-arginine supplementation ameliorates the glomerular filtration rate in renal transplant patients receiving grafts with a short cold ischemia time 24. Contrasting studies in transplant patients show that L-arginine supplementation has no effect on graft function and survival 25. L-arginine administration might be protective, although the outcome depends on factors like cold ischemia time and use of immunosuppression. NO donors such as molsidomine, sodium nitroprusside (SNP) and nitroglycerin have been rarely used in human transplant conditions. However, they have been shown to be protective in murine renal ischemia-reperfusion models 26. Administration of nonspecific inhibitors of NOS like NG-nitro-L-arginine and L-NG-monomethylarginine aggravates allograft rejection by causing endothelial dysfunction, suggesting that eNOS-derived NO is protective during allograft rejection. Specific iNOS inhibition is protective in renal IRI 27. However, in experimental transplantation models a differentiation in iNOS modulation exists between grafts versus recipients. While pharmacological iNOS inhibition in recipients seems protective 28, iNOS knockdown in donor kidneys accelerates allograft loss 29. This suggests an ambiguous role for iNOS depending on external factors. Interestingly, nitrite, the stable end-product of NO, has renoprotective properties in murine models for brain dead (BD) and IRI, possibly via conversion to NO 5, 30. Furthermore, serum and urine NOx levels showed alterations during acute rejection, suggesting a possible role as biomarker in renal allograft rejection. Modulation of the NO system as a strategy against renal graft failure needs further exploration (Table 1).
| Model | Inducing/inhibiting agent | Outcome | Refs. | |
|---|---|---|---|---|
| NO | Rat—IRI | Nitrite | Attenuated renal dysfunction and injury | 48 |
| NO donors (SNP, FK409) | Increased survival, improved renal function, less renal damage | 26, 49 | ||
| iNOS inhibitor GW274150 | Improved renal function, less renal damage | 27 | ||
| NO inhibitor L-NAME | Aggravated renal injury | 49 | ||
| Rat—transplantation | L-arginine | Improved graft function, less renal damage | 50 | |
| NO inhibitor L-NAME | Increased graft dysfunction and renal damage | 50 | ||
| iNOS inhibitors (L-NIL, BAI) | Improved renal function, less renal damage | 28 | ||
| Rat—BD donor | Nitrite | Less renal damage, improved posttransplant renal function | 30 | |
| Pig—preservation | SNP | Increased renal blood flow | 51 | |
| Pig—DCD | SNP | Biphasic effects, initially beneficial, but later deleterious | 52 | |
| Human—transplantation | L-arginine | Increased GFR and renal plasma flow | 24 | |
| CO | Mouse—IRI | [Ru(CO)3Cl2]2, CORM-3 | Improved renal function, less renal damage | 53 |
| Rat—transplantation | CO gas | Increased recipient survival, improved graft function, less renal damage | 32, 35, 54 | |
| CO donors (CORM-2, CORM-3, methylene chloride) | Improved transplant survival and function, less renal damage | 34, 36, 55 | ||
| CO gas + biliverdin | Prolonged recipient survival, improved renal function, less renal damage | 45 | ||
| HO-1 induction (CoPP) | Increased survival, improved renal function, less renal damage, improved microcirculation | 56 | ||
| HO-1 inhibitor SnPP | Decreased survival and renal function, increased renal damage | 56 | ||
| Rabbit—IOPS | CORM-A1, CORM-3 | Improved renal function, improved mitochondrial respiration | 37 | |
| Pig—preservation/IOPS | CORM-3 | Increased renal blood flow, improved renal function | 51, 57 | |
| Pig—transplantation | CO gas | Improved graft function, less renal damage, accelerated recovery from DGF | 6, 58 | |
| H2S | Mouse—IRI | H2S inhalation | Improved survival, improved renal function, less renal damage | 14 |
| NaHS | Improved renal function, less renal damage | 10 | ||
| Rat—IRI | NaHS | Reduced renal injury and dysfunction | 17, 38 | |
| CSE inhibitor PPG | Impaired renal function, increased renal damage | 17, 39 | ||
| Rat—transplantation | NaHS | Increased graft function, less renal damage, increased recipient survival | 59 | |
| Pig—IRI | Na2S | Improved renal function, less renal damage | 60 | |
| Pig—aortic occlusion | Na2S | Attenuated organ dysfunction, less renal damage | 61 | |
| Pig—NHB donor | NaHS | Improved renal function, less renal damage | 40 |
- BAI, 7-butylhexahydro-1H-azepin-2-imine; BD, brain dead; CO, carbon monoxide; CoPP, cobalt protoporphyrin; CORM, CO-releasing molecule; CSE, cystathionine g-lyase; DCD, donation after cardiac death; DGF, delayed graft function; GFR, glomerular filtration rate; H2S, hydrogen sulfide; HO-1, heme oxygenase-1; iNOS, inducible nitric oxide synthase; IOPS, isolated organ perfused system; IRI, ischemia-reperfusion injury; L-NAME, L-NG-nitroarginine methyl ester; L-NIL, iminoethyl-lysine; NaHS, sodium hydrosulfide; Na2S, sodium sulfide; NHB, non-heart-beating; NO, nitric oxide; PPG, propargylglycine; SNP, sodium nitroprusside; SnPP, tin protoporphyrin.
CO in renal transplantation
The cytoprotective properties of HO-1 and its product CO mitigate injury in the transplanted kidney. HO-1 is up-regulated in several stressful events such as brain death, organ procurement and IRI 31, 32. Gene transfer-induced local HO-1 overexpression protects rat kidney transplants from IRI, acute renal allograft rejection and CRTF 33. Furthermore, there is an association between the HO-1 promoter polymorphism and renal transplantation outcome. Graft function is positively affected if the donor has a polymorphism that causes greater up-regulation of HO-1. HO-1 thus functions as a protective mediator during transplantation, suggesting beneficial effects of CO. Endogenously generated CO maintains the integrity of physiological function of organs. Exposure to low concentrations of CO (20–250 ppm) has cytoprotective effects equal to that attained by HO-1 induction in transplant-induced IRI due to its anti-inflammatory, vasorelaxant, immune-suppressant and anti-apoptotic properties 32, 34. Treatment of renal allograft recipients with inhaled low-dose CO effectively prevents development of CRTF, inhibits progressive CRTF and restores allograft function 6, 35. Furthermore, exposing kidney grafts to CO during cold storage seems to improve graft quality 32. A limitation of the clinical use of CO is its narrow therapeutic window. High concentrations (>100 ppm) are associated with toxicity due to interference with oxygen delivery. However, the clinical use of low doses of CO as donor treatment reveals beneficial effects on graft immunogenicity and CRTF 36. Moreover, to circumvent the risk of impaired oxygen delivery to organs and tissues, CO-releasing molecules (CORMs) have been created with reduced risk of toxicity due to high COHb levels. In a renal transplant model, CORMs revealed promising protective effects against IRI and improved early graft function and survival 34. Furthermore, CORMs improve mitochondrial respiration, which might also contribute to its protective properties 37. CO is a promising agent in the battle against diminished graft function, quality and survival (Table 1).
H2S in renal transplantation
Research on H2S and its producing enzymes CSE, CBS and 3MST mainly focuses on IRI. Changes in renal CSE and CBS expression and/or activity during ischemia and in the early phase of reperfusion suggest a role for H2S production in this process 10, 38. Administration of the CSE inhibitor propargylglycine (PPG) prevents recovery of renal function and integrity following IRI, supporting the pivotal role of endogenous H2S production by CSE in this process 39. Exogenous administration of H2S is investigated in experimental rodent and porcine models for renal IRI and transplantation (Table 1). Interestingly, treatment with H2S is protective in experimental models that are clinically applicable to donation after cardiac death (DCD) 17, 40, providing us with a possible tool to improve graft quality of suboptimal donors. Most studies investigating the effects of H2S in any physiological or pathological setting have exclusively used sulfide salts such as sodium hydrosulfide (NaHS) and sodium sulfide (Na2S), while few use gaseous H2S. An additional advantage of gaseous H2S is its ability to accurately regulate the concentration. A major limitation of the current donors is that H2S has a short half-life and its release is uncontrollable due to its volatile nature. Interestingly, Zhao et al 41 recently developed other H2S donors with a regulated release and showed their protective effects in a murine model of cardiac IRI. The mechanisms behind the protective effects of H2S therapy reside in its vasodilatory, anti-inflammatory, anti-apoptotic and anti-oxidant characteristics 10, 14. H2S targets different pathways to which these effects might be related. One of them is the induction of Nrf-2, thereby up-regulating endogenous anti-oxidant defenses 42. H2S is also able to support mitochondrial function, thereby contributing to the preservation of cellular energetics 1, 43. In contrast, renal and cardiac transplant tolerance is associated with a down-regulation of transsulfuration network genes like CSE. CSE controls T-helper-1 immune responses and thereby delays allograft rejection. In addition, CSE blockade resulted in a significant prolongation of heart allogeneic graft survival. However, a drop in CSE expression was observed after transplantation in rejected allografts from untreated recipients. The fact that CSE is associated with tolerance and is also present at rejection illustrates the coexistence of effector/regulatory mechanisms that determine immunologic fate of allografts 44. In line with the recent progress, H2S treatment shows promising effects in clinically relevant models for renal transplantation (Table 1).
Interactions Between Gasotransmitters in Renal Transplantation
Data on interactions between gasotransmitters in renal transplantation are scarce; however, there are some studies that address this subject in experimental IRI. Crosstalk between NO and H2S alleviated renal IRI-induced damage, and inhibition of excess NO release resulting from iNOS activation might be crucial in H2S-related kidney protection 38, 39. CO has the same effect as H2S on iNOS expression, since combined treatment with CO and biliverdin results in decreased iNOS expression and thereby attenuates renal IRI 45. Both H2S and CO treatment interfere in the NO production pathway by inhibiting iNOS, this being cardinal in reducing IRI. A combination therapy with H2S and CO delivery systems might even enhance the protective effect.
Another target might be the cGMP pathway since increasing cGMP levels is protective in murine models of IRI 46. Because gasotransmitters increase cGMP levels, this effect might be intensified by co-stimulation and H2S-mediated phosphodiesterase inhibition. An additional common feature of gasotransmitters relates to the induction of vascular endothelial growth factor (VEGF), which is a pivotal angiogenic and pro-survival factor that operates in concert with other factors to stimulate cell proliferation and differentiation, increase vascular permeability, and mediate endothelium-dependent vasodilation. The induction of VEGF is protective in models of renal disease by reducing renal fibrosis and preserving renal microvessel structure 47. The combined effects of gasotransmitters are still not well studied. One can only speculate that crosstalk between these gases may provide synergistic effects and additional modulatory control.
Conclusion
There is ample evidence supporting the multifaceted role of gasotransmitters during transplantation. Exogenous administration and endogenous manipulation of these gases are valuable tools in preventing transplant damage, since they play a pivotal role in the regulation of cell functions and in the reduction of tissue injury by activation of pro-survival pathways. Their ability to reduce oxidative stress, inflammation and apoptosis, preserve mitochondrial function, and stimulate vascular smooth muscle cell relaxation mitigates IRI during organ transplantation and thereby improves the quality of transplanted organs. Improving donor organ quality might result in an expanding pool of potential donors and reduce organ shortage. Future investigation is needed on the interaction of these gases and elucidating synergistic effects. It is important to recognize the need for the development of consistent dosing and ways of application in the transplant setting. The regulation, expression and function of these gaseous molecules are very complex, so optimal alterations in synthesis and activity are warranted. More clinical trials are needed to determine the indications, therapeutic doses and optimal times of administration as well as adverse effects. Additional value for the use of gasotransmitters in renal transplantation lies within its beneficial function in cardiovascular protection, which is a major issue in transplant patients. Although treatment with NO, CO and H2S is in its infancy, the features of these gases make them attractive therapeutical candidates in the transplant setting. Very recent work utilizing anti-inflammatory compounds capable of generating simultaneous H2S and NO supports this possibility.
Acknowledgment
This work was supported by a grant from the Dutch Kidney Foundation (NSN C08-2254).
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




