Galectin-3 in heart failure with preserved ejection fraction
Abstract
In the last decades it has been appreciated that many patients with heart failure (HF) suffer from HF with preserved ejection fraction (HFpEF). The diagnosis and treatment of HFpEF is difficult, as we lack specific markers of the disease and no specific treatments have been identified. Galectin-3 has a strong relationship to several aspects of the pathophysiology of HF, especially myocardial fibrosis, the transition from compensated to decompensated HF, and co-morbidities such as renal disease and diabetes. Many of these traits are very commonly observed in patients with HFpEF, and this suggests that galectin-3 may be particularly important and useful in the study of HFpEF. This review summarizes our knowledge of the role of galectin-3 in fibrosis, specifically in experimental models of HF and HFpEF. Galectin-3 may be a marker and also a causal factor, and experimental studies suggested that galectin-3 may be a target for therapy in HFpEF. The detrimental effects of aldosterone may, in part, be conferred via galectin-3, and there are data to suggest that aldosterone blockers are of more benefit in patients with high levels of galectin-3. Furthermore, the relationship of galectin-3 to clinical correlates of developing HFpEF in human subjects is discussed, and the association between increased levels of galectin-3 and new-onset HF and mortality in the general population is highlighted. Additionally, the usefulness of galectin-3 in patients with established HFpEF is described. We conclude that galectin-3 may be useful for early detection, phenotyping, risk stratification, and therapeutic targeting of individuals with early or established HFpEF in which fibrosis is a major contributor to the disease. Finally, we propose areas of further research that should validate the role of galectin-3 in HFpEF.
Heart failure with preserved ejection fraction
It is estimated that >50% of all patients presenting with signs and symptoms of heart failure (HF) suffer from HF with preserved ejection fraction (HFpEF).1,2 Within the past decades the relative proportion of patients with HFpEF compared with those with HF with reduced ejection fraction (HFrEF) has increased, suggesting an incremental importance of the disease.1,2 Higher age along with changes in risk factors in the community have contributed to this observation. Patients with HFpEF have several differences in clinical demographics when compared with patients with HFrEF: the typical phenotype is an older, obese female.1,2 Furthermore, they often have a greater prevalence of hypertension and AF.1,2 Overall, co-morbidities, with the exception of CAD, tend to be more frequent in patients with HFpEF, and controversy exists as to whether, for example, diabetes and renal dysfunction are less or more frequent.1–3 Community-based cohort studies have shown that mortality rates are high although not comparable in HFpEF vs. HFrEF,4,5 but results from large, prospective interventional trials point towards a better prognosis in HFpEF.6–9 Finally, the exclusion of certain co-morbidities as relevant modifiers of mortality in HFpEF may contribute to the observed distortion of prognostic data in interventional trials.5
The pharmacological treatment of HFpEF remains challenging. Antineuroendocrine therapies, albeit Class I recommendations in HFrEF, did not demonstrate clear outcome benefits in HFpEF: for ARBs, CHARM preserved did not demonstrate survival benefit, but a reduction in HF hospitalizations was achieved, albeit at a disputed cut-off for an LVEF of 40%.10 At a higher cut-off for LVEF (<45%; closer to current guideline recommendations11), no outcome benefit was observed in I-Preserve.8 For ACE inhibitors, only the prematurely terminated PEP-CHF trial is available, again without evidence for improved outcome.7 Using the mineralocorticoid antagonist (MRA) spironolactone, Aldo-DHF demonstrated benefits in cardiac reverse functional and structural remodelling, but was not designed as an outcome trial.12,13 Another trial testing spironolactone, TOPCAT, is currently ongoing, and outcome results are awaited for the end of 2013.14 For beta-blockers, data are sparse and inconclusive.15 As a result, none of these antineuroendocrine treatment strategies is currently recommended in HFpEF beyond established risk factor control.11
The reasons for failure of outcome trials in HFpEF are uncertain, but contributing factors include the following: (i) there is no unequivocally accepted definition of the disease, leading to diagnostic inaccuracy; (ii) the aetiology, pathophysiology, and phenotypic expression is heterogeneous; and (iii) incorrect therapies may have been used for inappropriate patient groups.
Definition
Heart failure with preserved ejection fraction is a syndrome, and the current definition used for large-scale clinical trials (i.e. HF signs and symptoms in the presence of a ‘preserved’ EF) is insufficient. Additional parameters, including biomarkers (natriuretic peptides) and imaging parameters (tissue Doppler, left atrial size, strain, and others), may improve the likelihood that HF symptoms are indeed of cardiac origin. However, most of these parameters are poorly validated for their use in clinical trials. In fact, there is no generally accepted diagnostic approach for HFpEF.11 As a result, there is an urgent need for new biochemical and imaging parameters that would enable physicians to assign cardiac dysfunction to the symptoms of the patient.
Aetiology, pathophysiology, and phenotypic expression
Heart failure with preserved ejection fraction is a syndrome with a number of underlying aetiologies and numerous pathophysiological alterations contributing to the heterogeneous disease state. Major pathophysiological factors implicated in the disease are impaired relaxation, increased LV stiffness and reduced compliance, atrial dysfunction, chronotropic incompetence, pre- and post-capillary pulmonary hypertension, and vascular stiffening.16 Low-grade inflammation and extracellular matrix accumulation and fibrosis are often described, and may significantly contribute to impaired LV filling, a hallmark of the disease. This large variability in phenotypic expression makes the success of a single pharmacological therapeutic approach for all HFpEF patients unlikely. Thus it is clear that there is a large unmet clinical need for better individual phenotyping of HFpEF patients. This would allow more targeted and individualized therapies. As an example, beta-blockers may have a role in HFpEF patients with inadequate sinus tachycardia, but may be deleterious in those with chronotropic incompetence. Similarly, antifibrotic therapies, e.g. by MRAs, might be most beneficial in those in an active profibrotic state. There is, again, an urgent clinical need for novel diagnostic procedures and markers that allow not only the diagnosis, but also a better phenotyping of the individual patient.
Wrong patients and wrong therapies
The simple definition of HFpEF used in clinical trials may have led to the inclusion of patients without true HF. In fact, in the echocardiography substudy of I-Preserve, a significant proportion of patients did not demonstrate structural or functional alterations of the heart. In addition, antisympathetic therapies and ACE inhibitors are most successful in systolic HF with marked neuroendocrine overactivity, a pathophysiological hallmark of HFrEF which is much less pronounced in HFpEF. As a consequence, the accepted benefits of antineuroendocrine therapies in HFrEF may not simply be mirrored in HFpEF.
The purpose of this article is to review the current knowledge on galectin-3, a β-galactosidase-binding lectin implicated in a chronic profibrotic process. Galectin-3 can be detected in the plasma, and has emerged as a novel biomarker for patients at risk for or with HF. Most importantly, galectin-3 could, for the first time, allow a more detailed phenotyping of HF patients into those with and without an ongoing profibrotic process resulting in collagen accumulation and progressive stiffening of the heart. This might, for the first time, prepare the ground for targeted therapeutic strategies in HFpEF.
Galectin-3: introduction and its relationship to heart failure
Galectin-3 is a β-galactoside-binding member of the lectin family in which 14 mammalian galectins have been identified. Galectin-3 is an ∼30 kDa protein and contains a carbohydrate recognition binding domain (CRD) of ∼130 amino acids that enables the specific binding of β-galactosidases and is encoded by a single gene, LGALSS3, located on chromosome 14, locus q21–q22. Galectin-3 has recently been linked to HF development17,18 and is implicated in a variety of processes that are thought to play an important role in the pathophysiology of HFpEF, such as myofibroblast proliferation, fibrogenesis, tissue repair, inflammation, and ventricular remodelling. In experimental studies, several rodent models of pressure overload, such as hypertension17,19,20 and mice subjected to aortic constriction,20 which resemble the human pathophysiology of HFpEF, as they present with LV hypertrophy, diastolic dysfunction, myocardial fibrosis, and ultimately HF, have shown significant increases in myocardial, renal, and vascular galectin-3 expression.
Plasma levels of galectin-3 may be measured with an FDA (Food and Drug Administration)-approved enzyme-linked immunosorbent assay (ELISA), and galectin-3 levels have been shown to correlate with HF severity.21–24 Recently, changes in galectin-3 over time were shown to have an important prognostic value in HF patients, with increases in galectin-3 independently associated with an increased risk of all-cause mortality and HF hospitalizations.25,26
Before galectin-3 can become a standard biomarker, there are several points, for instance its relationship to NT-proBNP and kidney function, which should be taken into account in the interpretation of galectin-3.27 However, most importantly, the vast majority of published articles on galectin-3 described patients with HFrEF, not HFpEF. Therefore, studies investigating the potential role and importance of galectin-3 as a biomarker in populations with HFpEF are warranted.
Heart failure with preserved ejection fraction: extracellular matrix and markers of extracellular turnover
A number of experimental observations have provided insight into the pathophysiological contribution of the extracellular matrix to HFpEF development. Studies have shown that HFpEF is characterized by an increase in cardiomyocyte stiffness,28 but also by production and deposition of extracellular matrix.29,30 Collagen production and metabolism are major players in the content and properties of the extracellular matrix and myocardial stiffness.30 It has been shown that collagen synthesis is enhanced in HFpEF, while collagen degradation is reduced.31 This is supported by the observation that circulating markers of collagen metabolism [e.g. N-terminal propeptide of collagen III (PIIINP) and C-terminal telopeptide of collagen I (CITP)] are increased in HFpEF patients, while markers of extracellular matrix turnover and matrix degradation such as matrix metalloproteinase 2 (MMP2) and matrix metalloproteinase 9 (MMP9) are reduced.31 Markers of extracellular matrix turnover are related to echocardiographic parameters of HFpEF.32 Cardiac fibrosis is difficult to quantify and in this regard more sensitive biomarkers that are easy to measure are important.33 Experimental studies in galectin-3 knockout mice have provided deeper insight into the action of galectin-3 in myocardial fibrogenesis, and a central role for galectin-3 in myocardial matrix formation has been proposed (Figure 1).
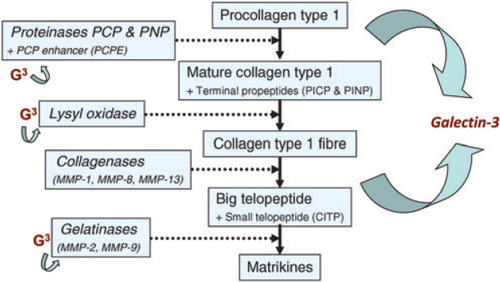
Experimental observations: galectin-3 in heart failure with preserved ejection fraction
Experimental studies suggest that galectin-3 is important for the development and progression of HFpEF. Sharma et al.17 studied a rat model of hypertensive HF with compensated LV hypertrophy (LVH), which later developed decompensated LVH as a model of HFpEF. The importance of galectin-3 in the pathophysiology of HF development in this rat model was further supported by their finding that galectin-3 was also up-regulated in biopsies from patients with severe aortic stenosis, a condition associated with cardiac functional and structural changes comparable with HFpEF. Furthermore, Sharma et al. demonstrated that infusion of galectin-3 induced severe LV fibrosis and LV dysfunction, suggesting that galectin-3 may be causally implicated in the pathophysiology for HF development and not just be a marker for disease progression. Others have confirmed these findings.34 In addition, several rodent models of pressure overload, such as hypertensive rats17,19,20 and mice subjected to aortic constriction,20 presented with substantial increases of myocardial, renal, and vascular galectin-3. Interestingly, these models resemble the human pathophysiology of HFpEF, as they present with LV hypertrophy, diastolic dysfunction, myocardial fibrosis, and ultimately with progression to LV dilatation and failure. In all stages of the disease, galectin-3 was found to be elevated. Specifically, galectin-3 co-localizes with sites of interstitial fibrosis, and shows a further increase at the stages of decompensation, when compensated LVH progresses into decompensated HF.17,20 The increase in galectin-3 may also be a target for therapy: in mice that are deficient for galectin-3, myocardial fibrosis is absent in pressure overload.20 Inhibition of galectin-3 with specific carbohydrates was associated with an attenuation of diastolic dysfunction, such as lower LV end-diastolic pressure and LV improved relaxation in a rat model, accompanied by an almost complete inhibition of LV fibrosis.20 Importantly, in an experimental model of cardiac hyperaldosteronism with angiotensin II-induced hypertension, the MRA eplerenone was able to decrease galectin 3 levels,35 an observation that was recently confirmed.19
Epidemiological observations: galectin-3 and risk for incident heart failure with preserved ejection fraction in the general population
Ho et al. investigated whether galectin-3 is a relevant marker linked to the development of HF in the general population.36 Galectin-3 concentrations were measured in 3353 participants in the Framingham Offspring Cohort and were associated with increased LV mass in age- and sex-adjusted analyses. During long-term follow-up (11.2 years), galectin-3, even after adjustment for clinical variables and BNP, was independently associated with an increased risk for incident HF [hazard ratio (HR) 1.23] as well as all-cause mortality (multivariable adjusted HR 1.15; P = 0.01). This confirmed a previous observation in 7968 subjects from another community-based cohort (PREVEND37). Of particular interest, in the study by Ho et al.36 there were no differences in baseline levels of galectin-3 and the risk for incident HFpEF vs. HFrEF, suggesting an important role for galectin-3 in the pathophysiology of HF, independent of LVEF.
Galectin-3 and clinical determinants of heart failure with preserved ejection fraction
The spectrum of patients at risk for the development of pre-clinical diastolic dysfunction or clinically manifest HFpEF is characterized by heterogeneity of phenotypic subtypes with potentially different pathophysiological processes resulting in different presentations, outcomes, and treatment options. Assuming that diverse pathways such as cardiac stress, inflammation, fibrosis, and tissue injury can be quantified, it can be envisaged that biomarkers should have an important role in risk prediction, diagnosis, and management of patients with HFpEF. Galectin-3 is a biomarker that reflects inflammatory and fibrotic processes and has been shown to be predictive of increased risk for the development of HF18 and adverse outcomes in patients with overt HFpEF.23
The heterogeneous pathophysiological nature of HFpEF can partly be related to the impact of classical risk factors and co-morbidities, such as hypertension, metabolic syndrome, and diabetes, as well as low-grade inflammatory states. This makes a biomarker that allows better diagnosis, phenotyping, and risk prediction in the HFpEF syndrome even more valuable. In fact, elevated galectin-3 levels may play a unique role in this clinical scenario, since galectin-3 is considered a direct mediator of HFpEF progression. A ‘galectin-3-positive phenotype’ may indeed predict the development of HFpEF in patients with co-morbidities.
In both large community-based cohorts in which galectin-3 was measured (PREVEND and Framingham Offspring, in total >10 000 subjects),37,36 plasma galectin-3 showed a strong relationship to blood pressure. The central role for blood pressure in the development of HFpEF is undisputed, and this interaction could contribute to the relationship between galectin-3 and HFpEF. Diabetes mellitus is common in HFpEF, and the interaction of diabetes with symptom burden and prognosis is more pronounced in HFpEF compared with HFrEF.1,5,38 In the general population as well as in patients at risk for HFpEF, diabetes mellitus was associated with elevated galectin-3 levels.36–37 Independent of the presence of diabetes, galectin-3 has been identified as a relevant important mediator of removal of advanced glycation end-products (AGEs) produced through the non-enzymatic glycation and oxidation of proteins known to be associated with phenotypic subtypes of HFpEF.39 These AGEs accumulate and contribute to myocardial stiffening mediated by AGE receptors;40 a correlation between serum AGE levels and development and severity of HF exists;40 and therapies targeted against AGEs may have therapeutic potential in HF.40 An AGE-specific cellular receptor complex (AGE-R) mediating AGE removal has been described, and related data suggest that galectin-3 interacts with AGE-R components and has an important role in modulating AGE-induced renal disease, suggesting a protective role for galectin-3 in the development of AGE-dependent tissue injury.41 Further studies are needed to understand fully the association of galectin-3 with AGEs in HFpEF.
There is evidence that galectin-3 is increased in obese patients, suggesting a possible interaction in the development of HFpEF.42 Obesity is a known risk factor of HFpEF, and also contributes to symptom burden, both in HFrEF and in HFpEF.38,43 Determination of whether increased galectin-3 levels themselves promote further proliferation of adipose tissue and whether galectin-3, obesity, and HFpEF are linked via the inflammatory adipose tissue needs to be addressed in future studies.44
Finally, in the general population, galectin-3 was correlated with renal function.37 Renal dysfunction is less common in HFpEF when compared with HFrEF, and only a few studies have investigated the link between galectin-3, the different aetiologies of HF, and renal function.23,27 In a small, retrospective study of patients with HFpEF, this association was addressed. The authors demonstrated that the relationship between galectin-3 levels and glomerular filtration rate (GFR) persisted after adjustment for age, LVEF, and NT-proBNP. Of particular interest, estimated GFR, rather than age, sex, or LVEF, was predictive of galectin-3 levels, suggesting that renal impairment might make a relevant contribution to the prognostic properties of galectin-3 in HF.27 Animal data showing that galectin-3 is related to the modulation of inflammation and fibrosis in the kidney also suggest an important link between renal function and HF occurrence.45 However, in other studies, the prognostic information driven by galectin-3 in HF still persisted after controlling for renal function. Thus, the impact of renal dysfunction on increased galectin-3 as a potential mediator of prognosis seems to be only one pathophysiological aspect.46
Clearly, renal galectin-3 may exert several direct actions, including stimulation of renal fibrosis.47 Of interest, galectin-3-deficient mice appeared resistant to salt loading as they do not increase their extracellular volume and blood pressure.48 Renal handling of galectin-3 remains largely unknown. Galectin-3 has a molecular weight of between 29 and 35 kDa, and therefore it is not likely that it is freely filtered.49 Furthermore, galectin-3 is a lectin and binds carbohydrates, and these galectin-3–carbohydrate aggregates may filter even less easily. However, galectin-3 can be measured in urine, and levels increase in patients with metastatic cancer.50 Although not investigated so far, ancillary mechanisms, such as electrical loading and tubular handling, may also play a role in renal handling of galectin-3. Therefore, mechanistic studies are needed to address renal handling of galectin-3 in more detail. Such future studies, more carefully focusing also on renal abnormalities in HFpEF, are needed to increase knowledge of the associations among galectin-3, renal dysfunction, and the symptom burden in these patients, as well as the confounding role of inflammation related to chronic diseases (arthritis, pneumonia) in HFpEF in the prognostic value of galectin 3.
Galectin-3 in heart failure with preserved ejection fraction
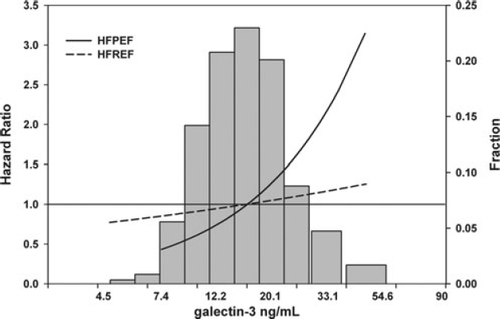
To date, only few clinical studies have described the levels and clinical correlates of galectin-3 in HFpEF. de Boer and colleagues23 studied 592 patients from the COACH study, a cohort of patients admitted with acute decompensated HF. Once stabilization and decongestion occurred, patients were prospectively enrolled in the COACH study, where 114 patients (19.2%) were found to have HFpEF. Absolute median values of galectin-3 were comparable between patients with HFpEF and HFrEF (∼20 ng/mL), while the HFpEF population was, as expected, older with a higher prevalence of females. While galectin-3 predicted worse outcome in the entire COACH population, patients with HFpEF had a much stronger correlation to galectin 3 levels than did those with HFrEF (Figure 2). Although very interesting, this observation should be interpreted with caution, because of post-hoc testing and the relatively small number of patients. However, there are several other observations suggesting that galectin-3 may have a more important role in HFpEF. The first study to identify galectin-3 as a predictive marker for HF was a substudy from the PRIDE study.21 The subjects enrolled in the PRIDE study were suffering from acute dyspnoea and, after careful adjudication, ∼200 of them were diagnosed with acute exacerbation of HF. In a subanalysis, it was shown that specific echocardiographic measures related to diastolic function, such as the tissue Doppler E/E' ratio, were particularly strongly related to galectin-3.51
Currently, there are no data regarding the relationship between galectin-3 levels and the response to specific pharmacological or non-pharmacological therapeutic approaches in HFpEF. This is in contrast to data available in HFrEF cohorts, where there is some evidence that baseline galectin-3 levels are predictive for the response to a specific drug therapy.25,52 These post-hoc analyses suggested that use of the study drug is less efficacious in patients with high galectin-3 levels. However, whether treatment effects of the antifibrotic drug spironolactone are linked to the galectin-3 level and its time-dependent course is currently being investigated in the Aldo-DHF trial.12
Summary and potential areas of research
Galectin-3 is a promising novel biomarker in the field of HFpEF. Due to its unique pathophysiological role in the profibrotic process, it may not only be a marker of disease severity, but also a mediator of disease progression. Therefore, it may prove clinically useful for early detection, phenotyping, risk stratification, and therapeutic targeting of those individuals where fibrosis is a major contributor to the syndrome. While this may preclude the use of galectin-3 levels as a first-line diagnostic marker for HFpEF, it is quite possible that someday it may be included in the classification of ‘Stage B HFpEF’. In other words, patients with structural abnormalities but no clinical HF are often difficult to classify. It is possible that the combination of natriuretic peptides and galectin-3 levels may yield a patient whose structural abnormalities include both increased wall stress and a propensity for fibrosis.
Thus, future research should include the establishment of whether galectin-3, alone or in combination with other disease-characterizing markers,53 has the potential to serve as a diagnostic marker for certain HFpEF states, and whether its prognostic power and risk stratification properties shown in acutely decompensated HFpEF patients is also evident in well compensated, clinically stable patients. Furthermore, data about the influence of galectin-3 levels on the response to different non-pharmacological and pharmacological therapeutic interventions, including antifibrotic substances such as MRAs, in HFpEF are currently lacking. This may identify a subgroup of patients with HFpEF that gain a greater therapeutic benefit in evidence-based HF therapies that have been to date disappointing in HF trials in the HFpEF population.54 The REGAL-HF (Reduction in Events with GALectin-3 in acute Heart Failure) trial is expected to be launched this year, and will test the hypothesis that patients with acute HFpEF and high galectin-3 levels will gain benefit from spironolactone (plus usual care) compared with patients treated only with usual care. Finally, whether the inhibition of galectin-3 itself has the potential to reduce cardiac fibrosis, to induce reverse cardiac remodelling, and to improve prognosis in patients with HFpEF should also be an area of future research in this field.
Conflicts of interest: BG Medicine Inc. (Waltham, MA, USA) has certain rights concerning the use of galectin-3 as a biomarker for heart failure. All authors report having received consultancy and/or speaker fees from BGM. The UMC Groningen, that employs R.A. de Boer, has received research grants from BGM.




