Safety and technique of ferumoxytol administration for MRI
Abstract
Ferumoxytol is an ultrasmall superparamagnetic iron oxide agent marketed for the treatment of anemia. There has been increasing interest in its properties as an MRI contrast agent as well as greater awareness of its adverse event profile. This mini-review summarizes the current state of knowledge of the risks of ferumoxytol and methods of administration. Magn Reson Med 75:2107–2111, 2016. © 2016 Wiley Periodicals, Inc.
OVERVIEW
In recent years, ferumoxytol, an intravenously (IV) administered ultrasmall superparamagnetic iron oxide agent marketed for treatment of anemia in adult patients, has attracted interest from the imaging community for a variety of clinical and research applications. Because of T1 shortening effects, long blood-pool residence time, and clearance through the reticuloendothelial system, ferumoxytol has been recently adopted for off-label clinical use as a vascular and nodal metastasis contrast agent, and as a research tool for studies involving macrophages and cell labeling. Furthermore, because ferumoxytol does not contain gadolinium, it may be an attractive alternative in those patients with renal failure who may be at risk of gadolinium-associated nephrogenic systemic fibrosis (NSF). Although ferumoxytol has a favorable premarketing safety profile, on March 30, 2015, the FDA strengthened its existing warning about the adverse event profile of ferumoxytol.
Associated Adverse Events and Relative Risk
Adverse Events in Postmarketing Clinical Trials
To date, postmarketing safety data are only available for therapeutic use of ferumoxytol. These include three multinational, randomized clinical trials 1-3 (n = 1094) and two nonrandomized studies 4, 5 (n = 8726). Most reported adverse events were mild, transient, and typically associated with the infusion process, although mild arthralgia/myalgia and headaches occurred up to 48 h postinfusion in one study 5. One study included 15 subjects with multiple drug allergies or asthma: These subjects received 125 mg methylprednisolone prophylactically. Aggregate adverse events are reported in Table 1A.
| A. Aggregate Adverse Events Reported in Postmarketing Safety Trialsa | |||
|---|---|---|---|
| Event type | n (total n = 10425) | Total percent | Percent range |
| Gastrointestinal | 174 | 1.74% | 0.6–12.5% |
| Headache | 57 | 4.21% | 1.8–13.3% |
| Muscle spasm/arthralgias | 40 | 2.96% | 1.5–23.3% |
| Cough/sneezing | 21 | 0.22% | 0.1–5% |
| Pruritis/rash/flushing | 68 | 0.68% | 0.4–10% |
| Dizziness | 56 | 0.56% | 0.2–5% |
| Dyspnea/chest pain | 48 | 0.48% | 0.2–5% |
| Hypersensitivity | 12 | 0.14% | 0.1–0.1% |
| Hypotension | 51 | 0.55% | 0.4–2.5% |
| Peripheral edema | 25 | 3.36% | 2.5–3.5% |
| Anaphylaxis | 3 | 0.03% | 0.02–1.3% |
| CCAEE | 9 | 0.89% | 0.8–1% |
| Urinary tract infections, nasopharyngitis | 39 | 5.67% | 5.4–7.5% |
| B. Ferumoxytol Administration Technique at Rates Concordant with FDA Recommendations; Some Applications May Benefit from Bolus Infusiona | ||
|---|---|---|
| Parameter | Recommendation | Examples |
| Indications | Vascular imaging, oncology (perfusion, nodal metastasis) | Vascular mapping in renal insufficiency |
| Formulation | 30 mg elemental iron/mL | |
| Full therapeutic dose | 14.6 mg/kg (two 7.3-mg/kg doses over 3–8-day period) | For 70-kg adult, full dose is 1020 mg (two vials, one vial given at a time separated by a few days) |
| MRI dose | 1–7.3 mg/kg | Venogram in 20-kg child using 3 mg/kg dose: 3 mg/kg * 20 kg = 60 mg (given 30 mg/mL formulation, 2-mL dose) |
| Dilution | 1 part undiluted ferumoxytol in 2–4 parts normal saline (ie, to concentration of no higher than 10 mg/mL) |
Example 1: 3 mg/kg dose in a 20-kg child: 60 mg = 2 mL ferumoxytol in 4-mL saline for a total of 6 mL Example 2: 3 mg/kg dose in a 100-kg patient: 3 mg/kg * 1 mL/30 mg * 100 kg = 10 mL ferumoxytol in 20-mL saline for a total of 30 mL |
| Infusion rate | Up to 0.5 mg/s | |
| Rapid bolus | Up to 2 mL/s (after dilution); NB: Not presently recommended by FDA | |
| Monitoring | Blood pressure, heart rate, and oxygen saturation before, 5 min after, and 30 min after administration | |
| Personnel | Physician with contrast reaction management training and ACLS and/or PALS certification in department; licensed nurse or physician in proximity to imaging suite | |
- a Hetzel et al (n = 406), Vadhan-Raj et al (n = 608), MacDougall et al (n = 80), Schiller et al (n = 8666), Auerbach et al (n = 60), Lu et al (n = 605) 52.
- Note: Although bolus infusion has been reported in several imaging publications, the FDA labeling for ferumoxytol advises against bolus infusion.
Serious adverse events included hypersensitivity 2, 4 and hypotension 4. The reported rates of anaphylaxis ranged from 0.02% with 2/8666 4 to 1.3% with 1/80 3, with a pooled aggregate rate of 0.03% (3/10425) based on published studies1-4. Reported deaths (n = 3) were considered unrelated to ferumoxytol 1, 2. The incidence of composite cardiovascular adverse event endpoint (CCAEE), which aggregates the incidence of a variety of cardiovascular adverse events including nonfatal myocardial infarction, heart failure, moderate-to-severe hypertension, and hospitalization resulting from any cardiovascular cause, was 2.7% 1.
Postmarket Surveillance
Since 2009, approximately 1.2 million therapeutic doses of ferumoxytol have been administered. In March 2015, the US Food & Drug Administration (FDA) Adverse Event Reporting System showed 79 anaphylactic reactions, with 18 fatalities despite immediate intervention. These deaths resulted in a boxed warning in March 2015 (http://www.fda.gov/Drugs/DrugSafety/ucm440138.htm). Twenty-four percent of these patients had multiple drug allergies, and nearly half of these anaphylactic reactions occurred within 5 min of administration. This rate of adverse events is lower than the rates initially reported in Phase II–III clinical trials.
Off-Label Imaging Use
To date, approximately 2000 patients across our institutions have received ferumoxytol for clinical MR imaging with standard monitoring procedures. We have had one case of an anaphylactoid reaction in a patient with multiple previous allergies who experienced diffuse cutaneous erythema (skin reddening) within seconds of starting a slow ferumoxytol infusion, followed by hypotension and delayed capillary refill. The infusion was stopped and the patient received IV fluids, IV diphenhydramine, IV ranitidine, and intramuscular epinephrine, resulting in resolution of erythema and hypotension. The MR scan was completed without further event. A literature search for ferumoxytol use in MRI performed in July 2015 revealed one report of a grade 2 allergic reaction 6. The reported patient had hives and throat swelling, associated with an infusion dose of 2.5 mg Fe/kg, which resolved with IV diphenhydramine. The imaging studies reviewed did not systematically evaluate for safety events.
Mechanism
IV iron administration, in general, can be associated with anaphylaxis and hypotension; ferumoxytol was specifically designed to minimize these risks. In Phase I–III studies, ferumoxytol demonstrated low immunogenicity 7, 8 and generated the lowest amount of labile-free iron compared with other IV iron therapies 9-11. Furthermore, ferumoxytol's isotonic formulation may partly explain the absence of adverse events related to rapid injection, unlike other iron preparations. Acute effects have been attributed to a combination of bioactive-free iron and mast cell release 12. Reaction recurrence can be mitigated by premedication with methylprednisolone, whereas nonsteroidal anti-inflammatory drugs can be used to prevent delayed arthralgias 12. Because diphenhydramine can cause somnolence, diaphoresis, hypotension, and tachycardia, premedication with diphenhydramine may actually worsen the acute response 13.
Risk Relative to Iodine- and Gadolinium-Based Contrast Agents
Risks of serious adverse events with ferumoxytol based on postmarket surveillance are similar to those associated with ionic iodinated contrast agents, and higher than those with gadolinium-based agents or nonionic iodinated contrast material 14, 15. However, the risks associated with iodinated and gadolinium-based agents in patients with severe renal disease (iodinated contrast-induced nephrotoxicity and NSF) are even higher and can be fatal 16, 17. Gadolinium deposits in deep nuclei of the brain 18 and delayed cases of NSF have been reported up to a decade after exposure 19, although the clinical significance of gadolinium deposition in the brain is unknown at this time.
Administration Practices
Work to date has largely been focused at single institutions, with fairly limited interaction among imaging centers. Administration details are inconsistently reported in the imaging literature. Based on the experience of the authors of this review, typical ferumoxytol doses for imaging range from 1 to 7.5 mg/kg, with most cases between 2 and 4 mg/kg. In most cases, a significantly smaller dose is given compared with the standard full therapeutic dose of 1020 mg (which is two doses of 510 mg and equates to 14.6 mg/kg for a 70-kg adult) for treatment of anemia, and reported doses are clustered around the 4 mg/kg recommendation produced by the seminal preclinical study by Prince et al 20. All reporting groups dilute the administered dose to a total volume of 24–60 mL for adults using saline. Injection rates at least partly reflect specific imaging indications, and range from a slow infusion (for lymph node and steady-state imaging) to bolus injections of 0.1–0.2 mg/(kg/s) for some angiographic applications 6, 21, 22. Higher injection rates and concentrations may be limited by artifacts from R2* effects 23, 24. Additionally, the FDA explicitly recommends a slow infusion of a diluted agent (Table 1B); therefore, a careful assessment of specific benefits and risks of bolus administration should be undertaken. Of note, MR image contrast may be altered by ferumoxytol for days to months after administration, whether for therapeutic or diagnostic purposes.
Evaluation for preexisting iron overload, absent in the imaging literature, is undertaken only by a minority of our groups, either through liver R2* measurements or serum ferritin levels. Most, though not all, reporting participants monitor patients for reactions for at least 30 min postadministration, including heart rate, blood pressure, and oxygen saturation. Administration is typically in a hospital setting, in which equipment and trained personnel for managing contrast reactions are readily available.
Ferumoxytol is distributed in a single-use vial containing 510 mg of iron. For multiple uses, the agent should be withdrawn and diluted in a sterile hood by trained pharmacy personnel. Typically, once a vial is opened, because of concerns about sterility, the final dose should be administered within 4 h after opening the vial. Although costs vary by region, ferumoxytol is generally more expensive than gadolinium agents; thus, the challenge of properly obtaining multiple uses from a single vial may be worth addressing in consultation with the institutional clinical pharmacy. However, in certain populations such as those with severely impaired renal function, in which no other options exist, the value of the diagnostic information obtained would outweigh the cost factor.
Similar to imaging procedures that involve ionizing radiation and off-label use of iodinated or gadolinium-based contrast agents, some of our clinical practices do not obtain informed consent for ferumoxytol administration 25, 26. As has been reviewed in this article, the primary risk is anaphylaxis, which is a rare occurrence. Currently, for clinical off-label use of iodinated and gadolinium-based contrast agents, there is no consensus as to whether consent should be obtained.
Potential Clinical Uses
Vascular Imaging
Ferumoxytol has been used in magnetic resonance angiography (MRA) of abdominal aortic aneurysms, evaluation for endoleaks after stent-graft repair of aneurysms, and assessment for renal artery stenosis 27-30. Given the known risks of gadolinium-based agents in patients with significant renal impairment, ferumoxytol has also been reported for renal transplant MRA 31, 32. Noncontrast MRA and venography techniques should be considered as alternatives in these settings, balancing the speed, resolution, and reliability of the various approaches. Additionally, several groups have described the use of ferumoxytol for the imaging of deep vein thrombosis and pulmonary thromboembolism 33-36, as well as pediatric cardiovascular imaging 37, 38. Quantitative first-pass perfusion MRI has also been described 39, 40, along with blood signal suppression for lymphangiography 41.
Macrophage Imaging
Macrophage uptake of ferumoxytol allows the assessment of macrophage migration and localization. Thus, the immune response to brain tumors, such as gliomas and lymphomas, may enable the imaging of tumor extent 22, 42. Similarly, ferumoxytol uptake has been associated with instability and impending rupture of vascular lesions, including intracranial aneurysms, arteriovenous malformations, and carotid plaques, based on macrophage localization 43-47. Finally, lymph nodes replaced by metastatic tumor will show reduced or absent uptake, although this is better established for other iron-based agents 48-51.
SUMMARY AND RECOMMENDATIONS
Ferumoxytol, although approved as a therapeutic agent, may be useful as an MRI contrast agent. Potential users should consider guidance from their institution's pharmacy committee before off-label clinical use. For research, investigators should seek the guidance of their local ethics board or institutional review board, and perform research studies only under approved protocols. Although the risk of acute adverse events with ferumoxytol is likely higher than that of gadolinium-based agents, ferumoxytol has a strong safety profile and may provide unique diagnostic information. Furthermore, it may be a valuable alternative for patients with renal insufficiency who may be at risk for NSF should they receive a gadolinium-based contrast agent. Ferumoxytol, like iodinated and gadolinium-based contrast agents, should be administered in an environment in which trained personnel, monitoring equipment, and resuscitation supplies are immediately available. Just as with other contrast agents, additional caution should be exercised in patients with prior or multiple drug allergies.
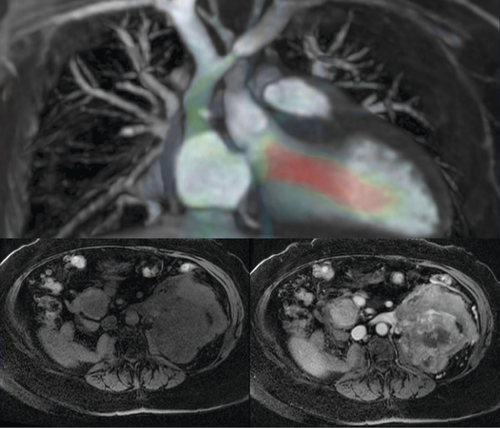
Representative applications include detailed cardiovascular imaging (top) and assessment of tumor perfusion through pre- (bottom left) and postcontrast (bottom right) imaging in a patient with a single kidney nearly replaced by metastasis.