Detection of plaque structure and composition using OCT combined with two-photon luminescence (TPL) imaging
Abstract
Background and Objectives
Atherosclerosis and plaque rupture leads to myocardial infarction and stroke. A novel hybrid optical coherence tomography (OCT) and two-photon luminescence (TPL) fiber-based imaging system was developed to characterize tissue constituents in the context of plaque morphology.
Study Design/Materials and Methods
Ex vivo coronary arteries (34 regions of interest) from three human hearts with atherosclerotic plaques were examined by OCT–TPL imaging. Histological sections (4 μm in thickness) were stained with Oil Red O for lipid, Von Kossa for calcium, and Verhoeff–Masson Tri-Elastic for collagen/elastin fibers and compared with imaging results.
Results
Biochemical components in plaques including lipid, oxidized-LDL, and calcium, as well as a non-tissue component (metal) are distinguished by multi-channel TPL images with statistical significance (P < 0.001). TPL imaging provides complementary optical contrast to OCT (two-photon absorption/emission vs scattering). Merged OCT–TPL images demonstrate the distribution of lipid deposits in registration with detailed plaque surface profile.
Conclusions
Results suggest that multi-channel TPL imaging can effectively identify lipid sub-types and different plaque components. Furthermore, fiber-based hybrid OCT–TPL imaging simultaneously detects plaque structure and composition, improving the efficacy of vulnerable plaque detection and characterization. Lasers Surg. Med. 47:485–494, 2015. © 2015 Wiley Periodicals, Inc.
INTRODUCTION
Despite continuing progress of optical imaging techniques in clinical diagnostics, therapeutics and intervention guidance 1-5, new imaging approaches are needed for improved efficacy of disease detection. In this study, we demonstrate a fiber-based hybrid optical coherence tomography and two-photon luminescence (OCT–TPL) imaging system to characterize atherosclerotic plaques in ex vivo human coronary arteries. The imaging system has advantages over OCT or TPL imaging alone, possessing two complementary types of optical contrast (i.e., two-photon absorption/emission and scattering). Further development of OCT–TPL imaging may provide cardiologists a diagnostic imaging tool that can simultaneously record co-registered images of plaque structure and biochemical composition, and improve the accuracy of vulnerable plaque detection.
The principal pathologic features of atherosclerotic plaques prone to rupture are well described. Davies et al noted that with the reconstruction of serial histological sections in individuals suffering from acute myocardial infarctions associated with death, a rupture or fissuring of “vulnerable plaque” was evident 6. Vulnerable plaques, recently defined by Virmani 7 using a more descriptive term “thin-cap fibroatheroma (TCFA)”, were further characterized as having a thin fibrous cap (typically less than 65 μm thick) overlaying a lipid core, increased infiltration of macrophages, and decreased smooth muscle cells compared to stable plaques 8-10. Many of the cellular/molecular events that lead to rupture of TCFAs, thrombus formation and consequent acute myocardial infarction are now understood and being utilized to develop novel imaging approaches and therapeutic interventions. Several features during atherogenesis have been identified that contribute to mechanical instability and increased risk of plaque remodeling and subsequent rupture such as infiltration and retention of low-density lipoprotein (LDL) binding to lipid 11, 12, a decrease in collagen synthesis 13, 14, and recruitment of macrophages 15, 16. Therefore, lipid content represents one of the important early biochemical markers that indicate and contribute to increased risk of plaque rupture in the coronary, cerebral, and peripheral circulations.
Intravascular OCT (IVOCT) is a catheter-based method for high-resolution imaging of blood vessels. The high axial resolution of IVOCT (e.g., 10 μm) has generated considerable interest as a candidate method to detect and assess atherosclerotic plaques (specifically TCFAs) 17. Fleg et al. reviewed the current clinically used imaging approaches for atherosclerosis diagnostics, including computed tomography (CT) angiography, magnetic resonance imaging (MRI), positron emission tomography (PET) for noninvasive characterization of plaque morphology; angioscopy, near-infrared spectroscopy, intravascular ultrasound (IVUS), IVMRI, and IVOCT for intracoronary plaque confirmation 18. Of all these imaging modalities, IVOCT is the sole approach that provides sufficient spatial resolution to image TCFA morphology. Although Yabushita et al. demonstrated a high sensitivity and specificity (94% and 92%, respectively) for lipid-rich plaque detection using IVOCT 19, 20, more clinically relevant, however, is the detection of TCFA with high sensitivity and specificity. Our group recently showed that IVOCT has a specificity of only 65% and a sensitivity of 100% for identification of TCFAs observed in 10 ex vivo human hearts (data not shown). This result is consistent with the 50% specificity and 100% sensitivity summarized from data derived from nine previously reported studies on TCFA frequency per coronary artery length using IVOCT or histology 21-29. In addition, obtaining sensitivity/specificity information using OCT relies on merely tissue structure (i.e., scattering properties of tissue) and subjective judgment from OCT image readers. Plaque composition and, hence, risk of plaque rupture, may not be accurately evaluated using OCT alone (i.e., indirectly inferring biochemical information from OCT depicted structures) because identification of lipid or calcium in plaques is based on darkness in the OCT image which can also be produced by a variety of light-tissue interaction mechanisms that result in an absence of backscattered photons 13. In comparison, TPL microscopy provides a complimentary contrast to OCT having the ability to image the actual biochemical information to improve the accuracy of TCFA detection, and has been utilized to directly characterize plaque composition including lipid, oxidized-LDL, calcium, and collagen/elastin fibers 30-33 based on endogenous auto-fluorescence signature from tissue with a superior spatial resolution (1 μm). However, this approach has been only applied outside the human body using a bench top microscope. The translation of traditional TPL microscopy into a catheter-based TPL imaging system for TCFA component characterization in vivo in combination with OCT for structural detection, is of potential clinical significance.
MATERIALS AND METHODS
Sample Preparation and Histology
For this study, three ex vivo human hearts were received from the South Texas Blood and Tissue Center. These hearts were de-identified, aside from basic clinical information, in compliance with the Institutional Review Board at the University of Texas Health Science Center in San Antonio, and informed consent for research was obtained from the donors' families. All three heart donors (male, 62, 55, and 73 years old) had a history of hypertension. Among these individuals, the cause of death was myocardial infarction (one; Heart1) and cardiac related (two; Heart2,3), respectively. Following excision of the hearts, the coronary arteries of each heart were flushed with 1 L of saline containing heparin. The right coronary artery (RCA) including the right coronary ostium, left anterior descending artery (LAD) including the left main artery and left coronary ostium, and left circumflex artery (LCX) were carefully dissected from the hearts. LCX was separated from the LAD at the bifurcation of left main artery. Following dissection, LAD, RCA, and LCX were filleted along the vessel axis to expose the vessel lumens. The harvested arteries were stored immediately in PBS at 4°C. Atherosclerotic plaques were identified by visual inspection and/or mechanical stiffness of the luminal surface (confirmed by histology). A total of 34 regions of interest (ROIs) on nine coronary arteries from three hearts with atherosclerotic plaques were found for hybrid OCT–TPL imaging to identify lipid and calcium. Specifically, two types of plaques were classified as either lipid rich (26 ROIs: 8, 10, 8 from Heart1-3, respectively) or calcified plaques (8 ROIs: 4, 1, 3 from Heart1-3, respectively). After imaging, ROIs at a field of view of 1.6 × 1.6 mm2 were marked with Indian ink on the lumen at the edges for subsequent histology. The time between death and OCT–TPL imaging was less than 72 hours.
The imaged tissue was processed routinely for histology. Coronary artery segments of the 34 ROIs were immersion-fixed in formalin and processed with standard freezing medium embedding. Arteries with substantial calcification were decalcified before embedding. Sections (4 μm in thickness) were cut at the marked imaging sites and stained with Oil Red O (ORO) 34, Von Kossa 35 and Verhoeff–Masson Tri-Elastic (Tri-Elastic) 36, markers for human lipid, calcium, and collagen/elastin fibers, respectively.
When excited with ultrashort pulsed irradiation, TPL emission spectra of metals are known to share a similar pattern with emission intensity increasing at longer wavelengths where the TPL excitation wavelength is located 37, 38. Metallic components such as gold nanoparticles used in targeted imaging approaches to identify plaque-based macrophages 39-42 and drug eluting stents with a metallic platform deployed during cardiovascular intervention 43 may also be present in coronary arteries. In this study, a convenient planar metallic sample (i.e., stainless steel ruler) was utilized to investigate the TPL emission spectrum difference between native plaque tissue and metallic constituent in coronary arteries.
Experimental Setup
A novel fiber-based hybrid OCT-TPL imaging system was constructed and utilized in this study, consisting of swept-source OCT and TPL imaging modules and scanning optics (Fig. 1). A swept-source laser (HSL-2000, Santec, Hackensack, NJ) centered at 1,310 nm with an 80 nm bandwidth and 20 kHz repetition rate was used to provide a broadband spectrum for the custom-built intensity OCT module. Average power incident on the sample was measured at 0.5 mW. Free-space OCT axial resolution was determined to be 20 μm (∼15 μm in tissue). A femtosecond Ti:Sapphire laser (Mai Tai HP, Newport, Irvine, CA) with excitation wavelength tunable over 760-1040 nm (80 MHz) was utilized in the custom-built TPL imaging module. Broadband TPL emission (490-680 nm) from the sample was divided into three channels and collected by three photomultiplier tubes (PMT1,2: H7422P-40, PMT3: R3896, Hamamatsu, Bridgewater, NJ) with associated band-pass filters in spectral ranges of 640–680 nm, 575–620 nm and 490–560 nm, respectively. TPL emission signals, from both plaque components including lipid/oxidized-LDL particles and calcium, and exogenous chromophores such as metal, were detected and distinguished by their relative intensities in the three PMT channels. To eliminate excitation laser light in the detection light path, a short-pass filter (et720sp, Chroma Technology, Bellows Falls, VT) was placed after TPL emission signal is separated from excitation by the dichroic mirror.
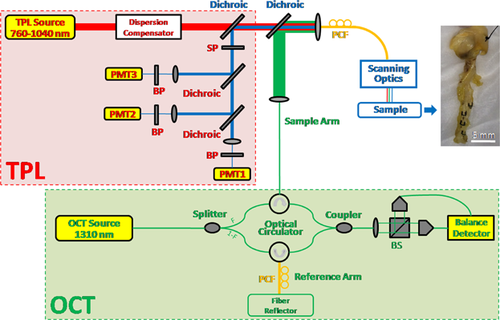
To combine OCT and TPL modules, two dichroic mirrors (760DCXRU, T970DCSPXR-XT, Chroma Technology, Bellows Falls, VT) were used to provide a common light path for OCT and TPL imaging. A 1 m long photonic crystal fiber (PCF, LMA-15, NKT Photonics, Birkerød, Denmark) with a 15 μm core diameter was used to simultaneously transmit single-mode OCT and TPL excitation/emission light to/from the sample. The scanning optics consisted of a doublet PCF collimator (f = 40 mm, Thorlabs, Newton, NJ), x- and y-galvanometers (6M2205S-S, Cambridge Technology, Bedford, MA), and a triplet scanning lens (f = 50 mm, Thorlabs, Newton, NJ). OCT and TPL excitation beam sizes (i.e., lateral resolution) on the sample are very similar and were determined to be ∼16 μm. The OCT–TPL image size was 512 × 512 pixels with a field of view of 1.6 × 1.6 mm2.
To maintain temporal transform-limited TPL excitation pulses on the sample after transmission through all optical elements including the 1 m PCF, a custom-designed adjustable dispersion compensator was utilized, consisting of a diffraction grating (1,200 lines/mm, Wasatch Photonics, Logan, UT), a focusing lens (f = 100 mm, Thorlabs, Newton, NJ), a polarizing beamsplitter (PBS122m, Thorlabs, Newton, NJ), a half-wave plate (WPH05M808, Thorlabs, Newton, NJ), a quarter-wave plate (WPQ05M808, Thorlabs, Newton, NJ) and a mirror (BB1-E03, Thorlabs, Newton, NJ). Pulse duration on the sample was measured to be 122 fs at 800 nm (the TPL excitation wavelength used in this study) with the dispersion compensator, compared to 119 fs at the output of the TPL excitation laser.
Statistical Analysis
Statistical analysis was performed using SPSS Software for Windows (Version 19.0.0, SPSS). A total of 34 ROIs of lipid rich and calcified plaques found on nine arteries from three hearts were all included in this study. Sample regions (i.e., plaque components) from each ROI were randomly selected based on their respective TPL emission spectrum and availability to eliminate selection bias. TPL emission from the samples (i.e., plaque components and metal) was then normalized to the channel with maximum intensity and was represented as mean TPL emission intensity for each channel (i.e., Channel1 to Channel3). One-way ANOVA analysis was performed for comparison of mean TPL emission intensities between samples for each channel. Additionally, Games–Howell Post Hoc Test 44 accounting for unequal group sizes/variances was performed to identify the difference of mean TPL emission intensities between each of the two samples by pair-wise comparison. A two-tailed P value less than 0.05 was considered statistically significant for both tests.
RESULTS
Multi-channel TPL Imaging of Atherosclerotic Plaque Components
We investigated the capability of TPL imaging to identify different biochemical constituents in atherosclerotic plaques. Lipid rich (26 ROIs) and calcified plaques (8 ROIs) were examined. A stainless steel ruler (5 ROIs) was also imaged for TPL emission spectrum comparison with the native tissue. Broadband TPL emission was separated into three channels at 490–560 nm (Channel3), 575–620 nm (Channel2) and 640–680 nm (Channel1), with PMT gains set the same for all three channels. Detected TPL emission signal was then normalized to the channel with the highest intensity.
Figure 2A and B show typical TPL images of plaques that contain lipid and calcium, respectively. Two characteristic lipid structures were identified in the TPL image of lipid rich plaque (Fig. 2A), including higher density and greater intensity bright spots (Lipid1, <50 μm in size) and lower density and reduced intensity larger oval-shaped bright regions (Lipid2, 50–250 μm in size), suggesting that TPL emission from these two structures may originate from different types of lipid formation (i.e., oxidized-LDL particles and lipid droplets/deposits, respectively). In contrast, a structure with a “rocky” appearance corresponding to bright TPL emission was detected in calcified plaque regions (Fig. 2B), distinct from lipid rich tissues. Histology with ORO and Von Kossa stains was performed on lipid rich and calcified plaques, respectively, to verify the presence of lipid and calcium. Figure 2D shows positive lipid stain on the lipid rich plaque. Specifically, high density red color droplets in depth are evident on the right side of the image while bulk homogeneous red color stains are visualized on the left side. This observation is consistent with characteristic lipid structures in the corresponding TPL image where Lipid1/Lipid2 dominates the right/left half of the yellow dashed line in Figure 2A. As the depth of focus of TPL excitation beam at 800 nm is about 500 μm, TPL emission signal (i.e., brightness in the TPL image) is integrated over the most superficial 500 μm tissue depth. Therefore, the signal may represent lipid distributed anywhere within this depth range in the corresponding histology image. Figure 2E shows positive calcium stain on the calcified plaque, suggesting that the structure with the “rocky“ appearance in the corresponding TPL image (blue dashed line in Fig. 2B) originates from calcium. In addition, a TPL image of a stainless steel ruler is shown in Figure 2C. TPL emission signal intensity versus PMT channels (i.e., wavelengths) for different plaque components and metal is plotted and compared in Figure 2F. TPL emission from Lipid1 and calcium shares a common trend that TPL signal intensity maximizes in Channel3 (490–560 nm), decreases in Channel2 (575–620 nm) and reaches minimum in Channel1 (640–680 nm); in comparison, TPL emission from Lipid2 increases (from Channel1 to Channel2) and then decreases (from Channel2 to Channel3); in case of metal, TPL emission increases from Channel3 to Channel1 and maximizes in Channel1, opposite to native tissue where Channel1 is the minimum.
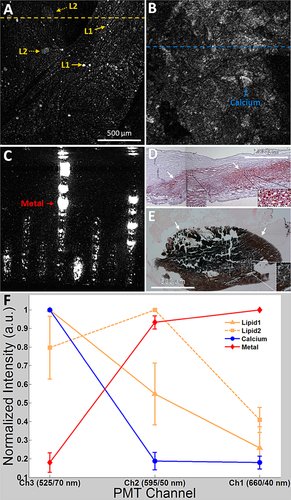
To further investigate the difference between the four signal types (i.e., Lipid1, Lipid2, calcium, and metal), a statistical analysis was performed on all three channels. A total of 75 Lipid1, 21 Lipid2, 10 calcium, and 5 metal sample regions (i.e., pixels in the TPL image) were randomly selected based on their respective TPL emission spectrum and availability in all ROIs. One-way ANOVA shows that mean TPL emission intensities between four signals for each channel (i.e., Channel1 to Channel3) are significantly different (P < 0.001). Games–Howell Post Hoc Test reveals the difference between any two of the four signals for each channel, as summarized in Table 1. Since a TPL emission spectrum is determined by combination of all three channels, two spectra are deemed different if mean values of TPL emission are significantly different in at least one channel. From this criterion, all four TPL emission spectra can be effectively separated with high statistical significance (P < 0.001).
| P Value | ||||
|---|---|---|---|---|
| Comparison Group | Ch3 (525/70 nm) | Ch2 (595/50 nm) | Ch1 (660/40 nm) | |
| Lipid1 | Lipid2 | <0.01 | <0.001 | <0.01 |
| Calcium | NA | <0.001 | 0.11 | |
| Metal | <0.001 | <0.001 | <0.001 | |
| Lipid2 | Calcium | <0.01 | <0.001 | <0.001 |
| Metal | <0.001 | 0.09 | <0.001 | |
| Calcium | Metal | <0.001 | <0.001 | <0.001 |
Hybrid OCT–TPL Imaging of Atherosclerotic Plaques
To demonstrate simultaneous detection of plaque structure and composition in human coronary arteries, hybrid OCT–TPL imaging was performed in this study. Co-registered OCT and TPL images were recorded and merged into a single image.
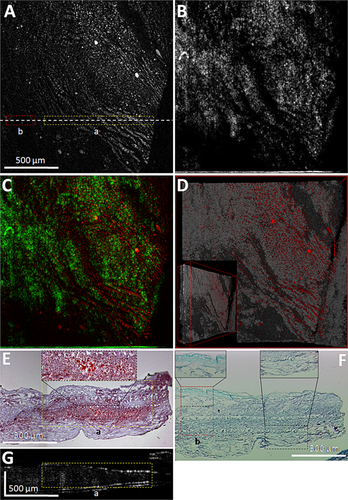
Figure 3A illustrates a TPL image of a lipid rich plaque region, TPL emission from isolated spots and curved elongated structures is visualized. Regions with strong TPL emission vary from 5 μm to over 500 μm (long axis), suspected to originate from either lipids and/or collagen/elastin fibers. TPL emission spectra in three channels at isolated spots (upper part of Fig. 3A) and curved structures (lower part of Fig. 3A) indicate that both follow spectral characteristics of Lipid1. Figure 3B shows a co-registered en face OCT image depicting the surface structure of the plaque region. Figure 3C is the merged OCT–TPL image that overlays biochemical composition onto the structural context of the plaque. Regions with strong/weak TPL emission correspond to weak/strong OCT signal, suggesting good complementarity of OCT and TPL signals due to two types of optical contrast (i.e., scattering and two-photon absorption/emission, respectively). A 3D OCT dataset is merged with corresponding TPL image (Fig. 3D), demonstrating chromophore distributions in relation to luminal surface profile of the plaque. To verify the origin of TPL emission signal, 4 μm thick tissue sections at the white dashed line in Figure 3A were cut after imaging for histology with ORO stain to detect lipid/oxidized-LDL particles (Fig. 3E) and Tri-Elastic stain to examine collagen/elastin fibers (Fig. 3F), respectively. Red color region (yellow dashed box in Fig. 3E) correlates well with higher TPL intensity regions in the yellow dashed box in Figure 3A, indicating the curved elongated structures originate from lipid/oxidized-LDL particles in the plaque. Green color regions (red dashed box in Fig. 3F) with evident collagen/elastin fibers give no TPL contrast in the red dashed box in Figure 3A, suggesting that TPL emission from collagen/elastin fibers may be very weak and may not contribute to the TPL signal intensity in this plaque at an excitation power density of 1.96 × 10−3 W/μm2. Figure 3G shows a B-scan OCT image at the white dashed line in Figure 3A, providing a cross sectional co-registration of the lipid region and OCT shadow region (yellow dashed boxes in Fig. 3E and G, respectively), also consistent with the region of strong TPL emission (yellow dashed box in Fig. 3A). Of note is that curved structures in the context of “bumpy” plaque surface profile in the 3D merged OCT–TPL image (Fig. 3D) indicate tissue regions consistent with fatty streak characterizations 45.
DISCUSSION
Two-photon luminescence (TPL) is based on two-photon absorption induced auto-fluorescence from tissue. Lilledahl et al. characterized TCFAs using TPL microscopy and second harmonic generation (SHG) 46. Results showed that TPL emission from calcium in the plaque exhibits a fluorescent peak at around 500 nm, consistent with our observation that TPL emission intensity of calcified plaque peaks in PMT Channel3 (490–560 nm). Yu et al. showed that TPL emission spectra of lipid droplets, foam cells and adipose tissue are similar and peak at 575–600 nm 47, suggesting that the origin of TPL emission from lower density and reduced intensity larger oval-shaped bright regions (i.e., Lipid2, 50–250 μm in size) in Figure 2A from lipid rich plaque, peaking in PMT Channel2 (575–620 nm), could represent either clustered foam cells (macrophages with intracellular lipid) and/or extracellular lipid droplets. Interestingly, Le et al. identified another lipid component in atherosclerotic plaques, oxidized-LDL, which has a broadband TPL emission and peaks at 525 nm 30, indicating that the TPL emission with a fluorescent peak in PMT Channel3 (490–560 nm) from higher density and greater intensity bright spots (i.e., Lipid1, <50 μm in size) in Figure 2A and Figure 3A from lipid rich plaque most likely arises from oxidized-LDL particles/aggregates. Detection and identification of different lipid sub-types such as foam cells/lipid droplets and oxidized-LDL particles provides a unique opportunity to evaluate plaque progression as oxidized-LDL taken up by foam cells can be hydrolyzed to release cholesterol and fatty acid into extracellular environment to become lipid droplets 48, 49.
CT, MRI, PET, IVUS, and fluorescence life-time imaging (FLIM) have been utilized in atherosclerosis diagnostics 18, 50, however, none of these approaches possess a high resolution comparable to OCT. Other imaging modalities including near-infrared fluorescence (NIRF) and single-photon emission CT (SPECT) are also undergoing clinical testing 51, 52, they require administration of exogenous imaging contrast agents. OCT has a high sensitivity (100%) in TCFA identification, however, our literature review suggested that the specificity is low (50%) 21-29. The hybrid OCT–TPL imaging in this study has the advantage of both OCT (high resolution) and TPL imaging (molecular sensitivity) and utilizes two different optical contrast mechanisms (i.e., scattering and two-photon absorption/emission), providing a more comprehensive understanding of tissue than using either technique alone and hence improving the accuracy of TCFA detection. For instance, lipid shows less backscattering than normal arterial tissue, and appears as weak signal regions in the OCT image (Fig. 3B and G). In contrast, lipid/oxidized-LDL particles gives rise to strong TPL emission in the same regions, depicting finer structures of lipid distribution (Fig. 3A). The overlay of OCT and TPL images with complementary optical contrast demonstrates simultaneously tissue structure and composition with a high resolution (Fig. 3C). Moreover, the known similarity between lipid rich and calcified plaque in OCT images, where both show lack of backscattering photons (i.e., both appear as shadow regions under luminal surface), has been problematic in plaque characterization 19. Lipid and calcium can be effectively distinguished by adding two-photon absorption/emission contrast which reveals biochemical information with high specificity, onto the context of tissue structure shown in OCT. In this study, hybrid OCT–TPL imaging was utilized for ex vivo plaque characterization. In vivo intravascular imaging can be achieved by replacing the scanning optics in the OCT–TPL system with a photonic crystal fiber (PCF)-based catheter similar in structure and mechanics to clinically used OCT catheters 53.
Although the fiber-based hybrid OCT–TPL imaging system is presented here for cardiovascular diagnostics, this system has the potential to be applied to other pathologies such as cancer (the second leading cause of death worldwide 54). Tumor-associated macrophages (TAMs) are known to play a fundamental role in the progression of many cancers (e.g., breast, prostate, ovarian, cervical, and cutaneous melanoma) 55. In vivo 3D nanoparticle-targeted TAM detection in the context of tumor structures (e.g., surface profiles, boundaries) using the novel hybrid OCT–TPL imaging may represent a powerful approach for cancer diagnostics.
Limitations
Although emission from chromophores at different depths (within 500 μm) is collected simultaneously, depth discrimination is not determined for the current TPL imaging system. Also, the power density (∼1.96×10−3 W/μm2) and numerical aperture (NA = 0.06) of TPL excitation laser on tissue samples through the current single-clad PCF was insufficient to excite and detect TPL emission from collagen/elastin fibers. However, identification of these features was demonstrated (data not shown) by traditional TPL microscopy with a high NA(=0.8) objective that provides a 8.7× higher laser power density (∼17.11 × 10−3 W/μm2). A double-clad PCF with a higher NA inner cladding (than that of the core) provides a much higher detection efficiency compared to single-clad PCF and, therefore, may enhance the detection of collagen/elastin fibers which are crucial components in fibrous cap concerning plaque vulnerability. Lastly, although the TPL emission spectra of plaque-based lipid, oxidized-LDL particles and calcium, and stainless steel can be effectively distinguished (P < 0.001) in this pilot study, the power of statistical analysis may be limited due to the single gender and small sample size (three hearts, all male) that may not represent the large population with atherosclerotic plaques. Results from this small subset of hearts should be validated by a larger study to confirm TPL emission spectrum difference between plaque components.
CONCLUSIONS
By utilizing multi-channel TPL imaging, plaque composition (i.e., lipid, oxidized-LDL particles and calcium) from human coronary arteries as well as non-tissue material (i.e., metal) can be identified and separated with a high specificity and statistical significance. Hybrid OCT–TPL imaging simultaneously detects plaque structure and biochemical composition in a co-registered and merged image, consistent with ORO and Von Kossa stains for lipid and calcium, respectively. Results of this study suggest that TPL imaging in combination with OCT provides a complementary optical contrast, having the potential to achieve a higher level of efficacy of vulnerable plaque detection and characterization.
ACKNOWLEDGMENTS
The authors would like to acknowledge the technical support from Chris Hoy, PhD and Jingjing Sun, ME. This work is supported by ASLMS Research Grant to Wang, Veterans Health Administration Merit Grant (I01 BX000397) to Feldman, American Heart Association Grant (13POST17080074) to Phipps, Research Grant from Clayton Foundation for Biomedical Research to Milner and Feldman, and Biomedical Engineering Advancement Fund from the University of Texas at Austin to Milner.




