Changes of ventricular and peripheral performance in patients with heart failure and normal ejection fraction: insights from ergometry stress echocardiography
Abstract
Aims
We assessed the left ventricular (LV) and peripheral performance at rest and during exercise in healthy and heart failure subjects with normal ejection fraction (HFNEF) or with reduced ejection fraction (HFREF).
Methods
All subjects received echocardiography at rest and with bicycle Ergometer exercise. The exercise images for two-dimensional speckle tracking were acquired with submaximal heart rate of 90–100 beats/min, while images for M-mode and tissue Doppler imaging were stored with attainment of >85% of predicted heart rate.
Results
A total of 80 HFNEF, 50 HFREF and 50 controls were studied. There was progressive decrease of two-dimensional global circumferential, radial and longitudinal strains (GCS, GRS and GLS), M-mode and tissue Doppler imaging long-axis parameters from controls, HFNEF to HFREF patients (all P < 0.05) at rest and on exercise. The degree of exercise-induced, long-axis augmentation (GLS and M-mode long axis excursion) decreased progressively from controls, HFNEF to HFREF subjects (all P < 0.05), while the increase in GCS and GRS was similar in all groups. The ventricular–arterial coupling ratio did not change in HFREF but reduced in HFNEF and controls during exercise (P < 0.01). All subjects had a similar resting heart rate, but patients exhibited chronotropic non-competence during exercise (P < 0.001).
Conclusions
Ventricular and peripheral dysfunction was evident in HFNEF at rest and deteriorated during exercise. The HFNEF patients had significantly impaired long-axis augmentation at stress that was intermediate between HFREF patients and controls. These findings have relevance to generation of symptoms on exercise in both HFNEF and HFREF.
Introduction
Heart failure is widely acknowledged as a complex clinical syndrome that impairs the ability of the ventricle to fill with or to eject blood, or to maintain a satisfactory delivery of oxygen to metabolizing tissue, especially on exercise. It is characterized by symptoms (dyspnoea and fatigue) and signs (evidence of a cardiac abnormality and particularly of congestion such as oedema, crackles).1 At least half of all patients with heart failure have a normal ejection fraction (HFNEF).1, 2 Similar to those with heart failure and reduced ejection fraction (HFREF), HFNEF patients have severe chronic symptoms, reduced exercise tolerance, and mortality.1, 2 Exercise intolerance is common in HFNEF patients. The mechanisms involved in exercise are complex and an individual's exercise capacity depends not only the response of ventricular and atrial function to exercise but also on the combined effects of arterial compliance, venous return, and autonomic function,3-6 and multiple factors likely interact to determine the degree of exercise limitation in HFNEF.
However, most HFNEF studies have only evaluated ventricular function at rest or exercise,3-9 and other mechanisms responsible for exercise intolerance, such as heart rate and vasodilation, have not been fully clarified, especially in comparison with HFREF. Accordingly, in the present study, using ergometry stress echocardiography, we examined multiple components of exercise limitation in HFNEF, including assessment of ventricular performance, myocardial contractility, chronotropic action, and vascular function, as well as ventricular–arterial interaction, and compared these with HFREF and normal patients.
Methods
Study population
A prospective cohort of patients with symptoms or signs of heart failure was screened. They were divided into two groups: HFNEF with left ventricular ejection fraction (LVEF) ≥50% who met the criteria of HFNEF according to the European Society of Cardiology (ESC) guideline,10 and HFREF with LVEF <50%. The asymptomatic healthy subjects with normal echocardiographic findings were included as controls.
All eligible patients were screened for other causes of heart failure and coexisting cardiac diseases. We excluded patients with acute coronary syndromes. Other exclusion criteria included: (i) atrial fibrillation, sick sinus syndrome, second or third degree heart block; (ii) congenital or valvular heart disease; (iii) infiltrative, restrictive, or hypertrophic cardiomyopathy; (iv) primary lung, renal, or hepatic disease; (v) obesity by Chinese standard (body mass index >25 kg/m2, and/or waist circumference >90 cm for men and >80 cm women);11 (vi) inability to exercise on an upright bicycle or to withhold cardiovascular medicines for 24 h; (vii) pregnancy; and (viii) suboptimal echocardiographic image. Beta-blockers were withheld for 24 h before the stress testing. The study was approved by institution's ethical committees and informed consent was obtained from all participants.
Study design
All subjects underwent symptom-limited (fatigue or dyspnoea) exercise testing on semi-recumbent and tilting bicycle Ergometer (Lode BV, Groningen, the Netherlands). Patient' heart rates (HR), brachial systolic and diastolic blood pressures (SBP and DBP, respectively), symptoms and 12-lead electrocardiogram (ECG) were monitored during exercise. The submaximal heart rate was 90–100 beats/min, and maximum exercise was defined by attainment of >85% of maximum age-predicted heart rate. Transthoracic echocardiography (Vivid7; GE Healthcare, Chalfont St Giles, UK) was performed with patients lying in the left lateral decubitus position during exercise. At least three sets of images with loops consisting of five consecutive cardiac cycles were stored for offline analysis with a designated software (EchoPac; Version BTO8 GE Vingmed, Horten, Norway).
Two-dimensional (2D) echocardiography
Resting LV dimensions were measured and LV mass was estimated and divided by the body surface area (BSA) to derive LV mass index (LVMI).12 The LVEF was calculated using biplane Simpson's method.12 The LV sphericity was measured by the LV short-to-long axis dimension ratio in end-diastolic apical four-chamber view. Left atrial volume was assessed by area–length method and indexed to BSA to derive left-atrial volume index (LAVI).12
Two-dimensional specking tracking echocardiography
The resting and submaximal exercise images were analysed offline using 2D speckle tracking echocardiography. The LV apical 4-, 2- and 3-chamber images and parasternal short-axis views at basal, mid and apical levels were used for assessing LV longitudinal, circumferential, radial strains and rotation. From standard 2D greyscale recordings, myocardial deformation was tracked frame by frame automatically within the region of interest bounded by the endocardial and epicardial borders throughout the cardiac cycle. Global strain was derived from the average of 18 segments in the longitudinal, or circumferential and radial planes—that is, six evenly divided segments in each of the three long-axis or short-axis views.13 Furthermore, LV twist was calculated as the net difference of peak systolic rotation between six basal and six apical segments.13
Tissue Doppler imaging (TDI) and M-mode echocardiography
The resting and maximal exercise images were analysed offline using TDI and M-mode echocardiography. The early (E), late (A) filling peak velocities, and deceleration time (DT) were measured from transmitral flow. Stroke volume (SV) was calculated using pulsed-wave Doppler, and indexed by BSA to derive the LV stroke volume index (LVSI). Cardiac index (LVCI) was determined from the product of LVSI and HR. The peak systolic (s′), early (e′), and (a′) late diastolic mitral annular velocities were also measured with pulsed-wave Doppler. The E/e′ ratio was derived from dividing the early mitral inflow velocity by the early diastolic mitral annular velocity. Furthermore, colour-coded tissue Doppler images for each of the six myocardial walls in LV apical views were acquired and systolic (Sm), early (Em), and late (Am) diastolic velocities were measured. The LV longitudinal reserve indices were calculated thus: systolic or diastolic longitudinal reserve index = ΔSm (or Em) × [1 – 1/Smrest (or Emrest)].14 In addition, using M-mode echocardiography, mitral annular plane systolic excursion (MAPSE) was estimated from apical four-chamber view.15
Derived parameters for evaluating LV contractility and vascular function
Mean arterial pressure (MAP) and pulse pressure were calculated thus: MAP = 2 × DBP/3 + SBP/3; pulse pressure = SBP – DBP.16 Load-independent myocardial contractility was assessed by preload recruitable stroke work index (PRSWI = MAP × SV/EDV) and single-beat end-systolic elastance (Ees = 0.9 × SBP/ESV).17, 18 Total afterload was determined by estimated arterial elastance (Ea = 0.9 × SBP/SV) and mean component of afterload by systemic vascular resistance index (SVRI = 80 × MAP/LVCI).16 Total arterial compliance (TAC) was estimated by the SV-to-pulse pressure ratio and the ventricular–arterial interaction by the coupling ratio (Ea/Ees).16
Statistical analysis
The sample size of the study was estimated from a small pilot study. For the change of LV twist with an expected mean difference of 5.3 and standard deviation 8.9, a sample size of 50 would provide 90% power with α = 0.01. SPSS version 17.0 was used for analysis (SPSS Inc, Chicago, IL, USA). Continuous and categorical variables were expressed as mean ± SD and percentages, respectively. Continuous variables between three groups were compared by analysis of covariance in a general linear model with LSD post-hoc analysis for subgroup comparisons, adjusted for age, gender, BSA and New York Heart Association (NYHA) class. The P-values after adjustment are presented. Categorical variables were compared by the chi-square test. Comparisons between rest and exercise in each subgroup were done by paired t-test. A value of P < 0.05 was considered significant.
Results
Subjects characteristics
According to protocol, we screened 227 consecutive patients with symptoms and signs of HF within 72 h of admission and 67 asymptomatic subjects with unremarkable clinical assessment and echocardiographic findings. Finally 180 eligible subjects (80 HFNEF, 50 HFREF and 50 controls) had sufficient exercise images for offline analysis. Figure 1 shows the recruitment flow chart in details.
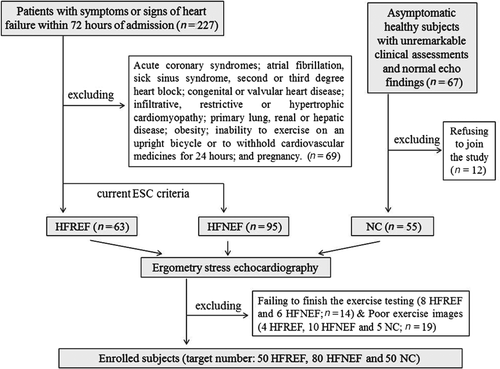
Patients were older with higher BSA than controls. Patient groups had comparable prevalence of hypertension, diabetes mellitus and anaemia except gender sex. All patients received similar medications and HFREF had higher NYHA class. Dyspnoea on exertion and pulmonary oedema were quite common in both HFNEF and HFREF, while muscle fatigue was more prominent in HFREF (P = 0.007). In HFNEF, 34% had hepatomegaly and 31% had ankle swelling, whereas these signs of fluid overload were present in more than two thirds of HFREF patients (P < 0.0005, Table 1).
| HFREF (n = 50) | HFPEF (n = 80) | Control (n = 50) | HFPEF vs. HFREF | |
|---|---|---|---|---|
| Age (years) | 62 ± 9b | 65 ± 8c | 56 ± 5 | NS |
| Male (%) | 90c | 64c | 35 | 0.001 |
| BSA (m2) | 1.72 ± 0.18a | 1.67 ± 0.17a | 1.61 ± 0.17 | NS |
| NYHA class (I/II/III/IV) | (0/27/17/6) | (33/36/11/0) | 0 | <0.0005 |
| Hypertension (%) | 56 | 46 | 0 | NS |
| Diabetes mellitus (%) | 36 | 28 | 0 | NS |
| Anaemia (%) | 6 | 5 | 0 | NS |
| Symptoms or signs | ||||
| Dyspnoea on exertion (%) | 94 | 78 | 0 | NS |
| Fatigue (%) | 96 | 60 | 0 | 0.007 |
| Hepatomegaly (%) | 72 | 34 | 0 | <0.0005 |
| Ankle swelling (%) | 68 | 31 | 0 | <0.0005 |
| Pulmonary oedema (%) | 88 | 79 | 0 | NS |
| Hospitalization for HF | 100 | 100 | 0 | NS |
| Medications | ||||
| ACEI/ARB (%) | 80 | 70 | 0 | NS |
| Beta-blockers (%) | 72 | 71 | 0 | NS |
| Calcium channel blockers (%) | 22 | 36 | 0 | NS |
| Diuretics (%) | 40 | 45 | 0 | NS |
| Statins (%) | 74 | 68 | 0 | NS |
| Digoxin (%) | 8 | 2 | 0 | NS |
| Aspirin (%) | 76 | 66 | 0 | NS |
| Standard resting echocardiographic parameters | ||||
| LVEDD (cm) | 5.6 ± 0.9c | 4.7 ± 0.5 | 4.4 ± 0.3 | <0.0005 |
| LVEDVI (mL/m2) | 97 ± 27c | 59 ± 14 | 53 ± 12 | <0.0005 |
| Biplane LVEF (%) | 36 ± 8c | 63 ± 6 | 65 ± 4 | <0.0005 |
| E/e' | 19.0 ± 10.6c | 14.1 ± 6.3b | 8.7 ± 1.8 | 0.002 |
| E/A | 1.86 ± 0.88c | 1.37 ± 0.25b | 1.14 ± 0.28 | 0.006 |
| DT (ms) | 171 ± 58c | 225 ± 54 | 217 ± 40 | <0.0005 |
| Ard-Ad (ms) | 42.3 ± 24.5c | 37.9 ± 23.3c | −17.3 ± 38.6 | NS |
| LVMI (g/m2) for male | 154.4 ± 36.5c | 125.3 ± 39.7c | 87.9 ± 21.3 | 0.001 |
| LVMI (g/m2) for female | 122.1 ± 23.3c | 105.3 ± 24.8c | 78.0 ± 15.1 | 0.007 |
| LAVI (ml/m2) | 36.9 ± 13.4c | 34.4 ± 13.2c | 23.6 ± 5.9 | NS |
| Sphericity | 0.62 ± 0.08c | 0.56 ± 0.07 | 0.54 ± 0.06 | <0.0005 |
| Exercise performance | ||||
| Exercise time (s) | 601 ± 283b | 660 ± 267b | 927 ± 288 | NS |
| Maximal workload (W) | 61 ± 19c | 65 ± 22c | 89 ± 23 | NS |
- ACEI, angiotensin-converting enzyme inhibitor; Ad, duration of mitral valve atrial wave flow; ARB, angiotensin receptor blocker; Ard, duration of reverse pulmonary vein atrial systole flow; BSA, body surface area; DT, deceleration time; E/A, ratio of early to late mitral inflow velocity; E/e′, ratio of early diastolic mitral inflow velocity to early diastolic mitral annular velocity; LAVI, left atrial volume index; LVEDD, left-ventricular end-diastolic diameter; LVEDVI, left-ventricular end-diastolic volume index; LVEF, left-ventricular ejection fraction; LVMI, left-ventricular mass index; HF, heart failure; HFNEF, heart failure with normal ejection fraction; HFREF, heart failure with reduced ejection fraction; NYHA, New York Heart Association. Comparisons were adjusted for age, gender, BSA and NYHA class when appropriate.
- a P < 0.05
- b P < 0.01
- c P < 0.001 vs. control.
Standard resting echocardiographic parameter and exercise performance
After adjustment for age, gender, BSA and NYHA class, HFNEF and controls had comparable resting LV end-diastolic diameter (LVEDD), LV end-diastolic volume index (LVEDVI), LVEF, DT, and sphericity index, and HFREF patients, as expected, had significantly larger LV chamber and volume, reduced LVEF, shorter DT, and increased sphericity than controls. Both patient groups had larger LAVI than controls (P < 0.001). The difference between duration of reverse pulmonary vein atrial systole flow (Ard) and mitral valve atrial wave flow (Ad) were comparable in patient groups, but significantly higher than controls (P < 0.001). The LVMI, E/e′ and E/A at rest were highest in HFREF followed by HFNEF and control groups (P < 0.01, Table 1).
Exercise time and the maximal achieved workload were similar between HFNEF and HFREF, but were significantly impaired in patient groups compared with controls (P < 0.01, Table 1).
Left ventricular global strain, twist and reserve
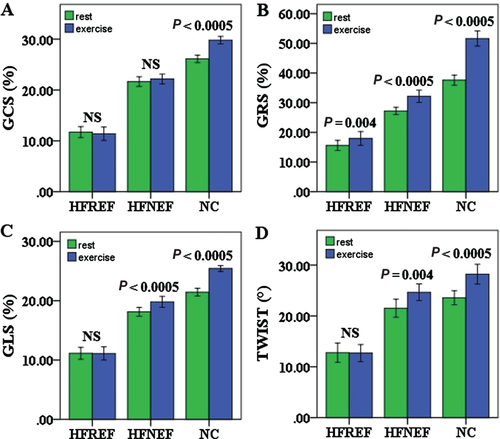
There was progressive decrease of 2D global circumferential, radial and longitudinal strains (GCS, GRS and GLS) from controls, to HFNEF to HFREF patients (all P < 0.001) at rest and during exercise. Moreover, the degree of exercise-induced augmentation of longitudinal function (GLS) decreased progressively from controls to HFNEF followed by HFREF (all P < 0.001), while the increase in circumferential and radial functions (GCS and GRS) was similar in all groups. Compared with controls, LV twist was similar in HFNEF group both at rest and during exercise, but was significantly decreased in HFREF group (P < 0.001, Table 2, Figure 2).
| HFREF | HFPEF | Control | HFREF vs. HFPEF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At rest | On exercise | P-value (paired t-test) | At rest | On exercise | P-value (paired t-test) | At rest | On exercise | P-value (paired t-test) | ||
| GCS (%) | 11.7 ± 3.8c | 11.4 ± 4.6c | NS | 21.7 ± 4.3c | 22.2 ± 4.3c | NS | 26.5 ± 2.3 | 29.8 ± 2.5 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔGCS (%) | −0.3 ± 2.6c | 0.5 ± 3.3c | 3.7 ± 2.6 | NS | ||||||
| GRS (%) | 15.6 ± 6.0c | 18.0 ± 8.1c | 0.004 | 27.2 ± 5.5c | 31.9 ± 9.4c | <0.0005 | 37.6 ± 5.9 | 51.6 ± 8.9 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔGRS (%) | 2.4 ± 5.7c | 4.7 ± 8.2c | 13.3 ± 4.6 | NS | ||||||
| GLS (%) | 11.1 ± 3.6c | 11.1 ± 3.8c | NS | 18.1 ± 3.4c | 19.8 ± 4.2c | <0.0005 | 21.4 ± 2.2 | 25.4 ± 1.7 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔGLS (%) | 0.1 ± 1.6c | 1.7 ± 2.4c | 4.0 ± 1.9 | <0.0005 | ||||||
| Twist (°) | 12.8 ± 6.7c | 12.7 ± 5.9c | NS | 21.5 ± 8.0 | 24.7 ± 7.4 | 0.004 | 23.6 ± 4.9 | 28.2 ± 6.9 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔTwist (°) | −0.1 ± 5.5 | 3.4 ± 8.4 | 4.4 ± 7.9 | NS | ||||||
| s' (cm/s) | 4.7 ± 1.5c | 7.3 ± 3.6c | <0.0005 | 7.0 ± 1.8b | 10.1 ± 2.8c | <0.0005 | 8.0 ± 1.3 | 13.0 ± 2.6 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| Δs' (cm/s) | 2.6 ± 2.9c | 3.0 ± 2.5b | 5.0 ± 2.4 | NS | ||||||
| e' (cm/s) | 4.4 ± 1.5c | 10.6 ± 3.7c | <0.0005 | 5.8 ± 1.6c | 12.9 ± 4.5c | <0.0005 | 8.6 ± 1.9 | 17.7 ± 3.9 | <0.0005 | <0.0005† |
| 0.015‡ | ||||||||||
| Δe' (cm/s) | 6.3 ± 3.6b | 7.0 ± 3.9a | 9.3 ± 4.2 | NS | ||||||
| Sm (cm/s) | 2.9 ± 0.7c | 4.5 ± 1.6c | <0.0005 | 4.6 ± 1.3c | 6.1 ± 1.7c | <0.0005 | 5.9 ± 0.5 | 8.8 ± 1.7 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔSm (cm/s) | 1.6 ± 1.2a | 1.5 ± 2.5b | 2.7 ± 1.6 | NS | ||||||
| Em (cm/s) | 3.4 ± 1.2c | 7.1 ± 2.1c | <0.0005 | 4.4 ± 1.1c | 9.0 ± 2.2c | <0.0005 | 6.6 ± 1.0 | 12.6 ± 2.6 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔEm (cm/s) | 3.7 ± 2.3c | 4.6 ± 2.1a | 5.9 ± 2.2 | NS | ||||||
| Systolic reserve index | 1.0 ± 0.9b | 1.1 ± 2.2b | 2.3 ± 1.3 | NS | ||||||
| Diastolic reserve index | 2.2 ± 1.3c | 3.4 ± 1.6c | 4.8 ± 1.9 | <0.0005 | ||||||
| MAPSE (mm) | 7.4 ± 1.8c | 8.2 ± 2.9c | <0.0005 | 10.7 ± 1.6c | 13.4 ± 2.8c | <0.0005 | 12.3 ± 1.6 | 17.5 ± 2.9 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔMAPSE (mm) | 0.8 ± 2.0c | 2.6 ± 2.5c | 5.1 ± 2.9 | 0.001 | ||||||
- BSA, body surface area; e′, early diastolic mitral annular velocity; Em, early diastolic myocardial velocity; GCS, global circumferential strain; GLS, global longitudinal strain; GRS, global radial strain; HFNEF, heart failure with normal ejection fraction; HFREF, heart failure with reduced ejection fraction; NYHA, New York Heart Association; MAPSE, mitral annular plane systolic excursion; s′, systolic mitral annular velocity; Sm, systolic myocardial velocity.
- Comparisons were adjusted for age, gender, BSA and NYHA class when appropriate.
- a P < 0.05
- b P < 0.01
- c P < 0.001 vs. control.
- † HFREF vs. HFNEF at rest,
- ‡ HFREF vs. HFNEF on exercise.
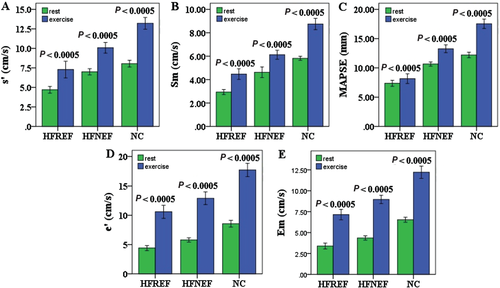
Left ventricular longitudinal function and reserve
Similar to our findings for LV global strain, there was progressive decrease of TDI Sm, s′ and M-mode MAPSE from controls, to HFNEF to HFREF patients both at rest and during exercise (all P < 0.01, Figure 3A,B,C). In diastole, TDI Em and e′ reduced progressively from HFREF, to HFNEF to controls at rest and this phenomenon was exaggerated during exercise (all P < 0.001, Figure 3D,E). Moreover, there was a progressive decline in augmentation of MAPSE during exercise from control, to HFNEF to HFREF groups (P < 0.001). HFNEF had similar reduced increase in TDI Sm, Em, s' and e′ as HFREF during exercise, and these parameters failed to rise normally during exercise (all P < 0.05). Additionally, patient groups had reduced longitudinal reserve indices than controls (Table 2).
Left ventricular contractile function and reserve
The PRSWI, a load-independent index for chamber contractility, decreased progressively from controls, to HFNEF to HFREF both at rest and during exercise (P < 0.01). HFNEF and controls had similar Ees at rest and HFREF had significantly lower Ees (P < 0.001), while during exercise Ees showed decreasing progressively from controls, to HFNEF to HFREF (P < 0.001). Moreover, the change of PRSWI and Ees decreased progressively from controls to HFNEF followed by HFREF (Table 3).
| HFREF | HFPEF | Control | HFREF vs. HFPEF | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At rest | On exercise | P-value (paired t-test) | At rest | On exercise | P-value (paired t-test) | At rest | On exercise | P-value (paired t-test) | ||
| PRSWI (mmHg) | 35.6 ± 8.8c | 43.3 ± 13.9c | <0.0005 | 55.4 ± 8.3a | 82.5 ± 11.8b | <0.0005 | 61.8 ± 7.7 | 92.6 ± 10.7 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔPRSWI (mmHg) | 8.3 ± 10.1c | 22.5 ± 12.5b | 31.1 ± 9.6 | <0.0005 | ||||||
| Ees (mmHg/mL) | 1.7 ± 0.9c | 2.1 ± 1.0c | <0.0005 | 4.7 ± 2.3 | 7.3 ± 3.2c | <0.0005 | 4.9 ± 1.2 | 9.3 ± 2.0 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔEes (mmHg/ml) | 0.4 ± 0.5c | 2.6 ± 2.2c | 4.4 ± 1.6 | <0.0005 | ||||||
| Ea (mmHg/ml) | 2.9 ± 0.9 | 3.8 ± 1.2 | <0.0005 | 2.6 ± 0.9 | 3.4 ± 1.1 | <0.0005 | 2.6 ± 0.6 | 3.6 ± 0.7 | <0.0005 | NS† |
| NS‡ | ||||||||||
| ΔEa (mmHg/mL) | 0.9 ± 1.0 | 0.8 ± 0.7 | 1.0 ± 0.7 | NS | ||||||
| SVRI (dyne · s/cm5.m2) | 3667 ± 1208a | 2688 ± 1056c | <0.0005 | 2657 ± 706a | 1938 ± 473 | <0.0005 | 3134 ± 650 | 1748 ± 533 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔSVRI (dyne · s/cm5.m2) | −842 ± 794 | −711 ± 650a | −1175 ± 691 | NS | ||||||
| TAC (mL/mmHg) | 0.89 ± 0.40 | 0.59 ± 0.26 | <0.0005 | 0.92 ± 0.45 | 0.58 ± 0.33 | <0.0005 | 0.96 ± 0.26 | 0.53 ± 0.15 | <0.0005 | NS† |
| NS‡ | ||||||||||
| ΔTAC (mL/mmHg) | −0.31 ± 0.35 | −0.37 ± 0.34 | −0.43 ± 0.26 | NS | ||||||
| Ea/Ees | 1.93 ± 0.91b | 2.07 ± 1.00c | NS | 0.61 ± 0.16 | 0.52 ± 0.19 | <0.0005 | 0.53 ± 0.09 | 0.40 ± 0.11 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔEa/Ees | 0.13 ± 0.70b | −0.09 ± 0.16 | −0.15 ± 0.10 | 0.007 | ||||||
| SBP (mmHg) | (R1) 135 ± 16a | 191 ± 29 | <0.0005 | 136 ± 19a | 196 ± 23 | <0.0005 | 126 ± 15 | 193 ± 23 | <0.0005 | NS† |
| NS‡ | ||||||||||
| ΔSBP (mmHg) | 56 ± 25 | 61 ± 23 | 67 ± 19 | NS | ||||||
| DBP (mmHg) | (R2) 79 ± 10 | 96 ± 21 | <0.0005 | 75 ± 11 | 98 ± 13 | <0.0005 | 77 ± 10 | 95 ± 13 | <0.0005 | NS† |
| NS‡ | ||||||||||
| ΔDBP (mmHg) | 17 ± 20 | 13 ± 13 | 18 ± 13 | NS | ||||||
| HR (bpm) | (R3) 69 ± 10 | 131 ± 21c | <0.0005 | 69 ± 13 | 123 ± 18c | <0.0005 | 64 ± 9 | 148 ± 11 | <0.0005 | NS† |
| NS‡ | ||||||||||
| ΔHR (bpm) | 61 ± 22c | 54 ± 18c | 84 ± 12 | NS | ||||||
| LVSI (mL/m2) | (R4) 29.0 ± 10.7b | 33.1 ± 13.1b | 0.011 | 37.3 ± 11.0 | 43.2 ± 10.7 | 0.006 | 38.5 ± 7.6 | 45.4 ± 9.4 | <0.0005 | <0.0005† |
| <0.0005‡ | ||||||||||
| ΔLVSI (mL/m2) | 4.2 ± 7.4 | 5.8 ± 6.9 | 6.6 ± 7.1 | NS | ||||||
| LVCI (L/mL.m2) | (R5) 2.4 ± 0.7b | 3.7 ± 1.5c | <0.0005 | 3.4 ± 0.9 | 5.4 ± 1.2 | <0.0005 | 3.5 ± 0.5 | 6.0 ± 1.3 | <0.0005 | <0.0005† |
| <0.001‡ | ||||||||||
| ΔLVCI (L/mL.m2) | (R6) 1.3 ± 1.2c | 1.9 ± 0.9c | 2.5 ± 1.2 | NS | ||||||
- BSA, body surface area; DBP, diastolic blood pressure; Ea, effective arterial elastance; Ees, end-systolic elastance; HR, heart rate; HFNEF, heart failure with normal ejection fraction; HFREF, heart failure with reduced ejection fraction; LVCI, left ventricular cardiac index; LVSI, left ventricular stroke volume index; NYHA, New York Heart Association; PRSWI, preload recruitable stroke work index; SBP, systolic blood pressure SVRI, systemic vascular resistance index; TAC, total arterial compliance.
- Comparisons were adjusted for age, gender, BSA and NYHA class when appropriate.
- a P < 0.05
- b P < 0.01
- c P < 0.001 vs. control.
- † HFREF vs. HFNEF at rest,
- ‡ HFREF vs. HFNEF on exercise.
Vascular function and reserve
There was no significant difference in arterial elastance or compliance (Ea and TAC) at rest and during exercise. Exercise-induced reduction in SVRI was seen in patients and controls, but the degree of the change was not different in all groups. The coupling ratio (Ea/Ees) was similar between control and HFNEF but was significantly higher in HFREF than control both at rest and during exercise (P < 0.01). Specifically, Ea/Ees did not differ significantly from rest to exercise in HFREF, whereas it showed significant reduction during exercise in HFNEF and control (P < 0.01, Table 3).
Haemodynamics and reserve
Patients had higher resting SBP than controls (P < 0.05), but during exercise SBP was similar in all groups. The resting and exercise DBP were comparable between patients and controls. Even though all subjects had a similar resting HR, patients failed to achieve a significant HR increase during exercise compared with controls (P < 0.001, Table 3). After adjustment for demographics, HFNEF and control groups had similar LVSI and LVCI at rest and during exercise, but HFREF patients had significantly reduced LVSI and LVCI (P < 0.01, Table 3).
Discussion
In this study, we comprehensively evaluated the LV and non-diastolic peripheral factors performances with stress echocardiography in HFNEF, compared with HFREF and normal control. We have demonstrated that a range of abnormalities of ventricular and peripheral mechanisms exist in HFNEF, including impairment of global LV circumferential, radial and longitudinal strain, reduced systolic and diastolic longitudinal function and reserve, reduced chamber contractility and chronotropic incompetence. Moreover, we found that HFNEF patients had a lesser degree of impaired exercise-induced, long axis augmentation (GLS and MAPSE) than HFREF patients. This paper extends the work on resting mechanics in our previous publication.19
Ergometry stress echocardiography for exploring exercise intolerance in HFNEF patients
Exercise-related symptoms are often the reason that HFNEF patients are referred for specialist assessment. What explains exercise intolerance in HFNEF? Previous studies have concentrated on measures of ventricular stiffness and delayed diastolic filling as being the primary reasons for higher ventricular diastolic pressure.2, 8 Consequently, increased diastolic pressures would raise pulmonary venous pressures, lung blood volumes and trigger dyspnoea. However, this concept stems largely from the studies performed at rest.2, 8 However, recent studies have now suggested that many of the abnormalities of ventricular and atrial function only become apparent on exercise when the patient becomes symptomatic.3-6, 20
Ergometry stress echocardiography with a supine bicycle can be used for cardiac diseases where an assessment of cardiac and haemodynamic response to physiological exercise is wanted. This non-invasive method provides important diagnostic information and improved understanding of pathophysiological mechanisms. We confirmed in the present study that one of the most obvious abnormalities is the failure of longitudinal function to increase normally on exercise, as indicated by the reduced GLS and MAPSE on exercise in HFNEF compared with controls and, potentially, this could be used as a diagnostic marker.21
Ventricular mechanics in HFNEF patients during exercise
We assessed LV global multidirectional strain and twist, longitudinal function and corresponding reserve to explore the ventricular mechanisms responsible for exercise intolerance in HFNEF. We demonstrated progressive impairment in global LV strain in different directions and LV systolic and diastolic longitudinal function from control, to HFNEF to HFREF groups both at rest and during exercise, confirming the findings of recent studies by Tan et al.6 and Wang et al.20 Moreover, the magnitude of increase in these parameters from rest to exercise was consistently lower in HFNEF compared with control, but this impairment of long axis augmentation (GLS and MAPSE) at stress was less severe than in HFREF patients. Thus it appears that there is a spectrum of myocardial dysfunction in heart failure patients, with HFNEF patients having a significantly impaired LV myocardial deformation and long axis function even at rest, and more so during exercise compared with controls, but myocardial longitudinal reserve in HFNEF patients was less reduced than in HFREF patients. This may reflect the severity and type of myocardial disease. These findings also confirm and extend some recent work that studied the clinical significance of long axis reserve between rest and exercise in HFNEF patients, as the extent of long axis impairment during exercise may play a role in the symptomatology of these patients.6, 20, 21 Thus, it is likely that the impairment of systolic function, in particular the impaired increase in longitudinal function, leads to impaired early diastolic filling and hence the rise in end-diastolic pressure.6, 22, 23 Any increased ‘stiffness’ measured at end-diastole is clearly not the only explanation. Like Wang et al.20 but in contrast to Tan et al.6 we found a discordance between the relatively preserved LV overall twist and severely reduced longitudinal function (MAPSE, GLS, Sm, Em, s′, and e′') in HFNEF. However, there was a wide scatter in torsion, with some patients having high values and others lower. This has been noted before and may be explained by the subendocardial and subepicardial fibres representing two oppositely directed spirals.24 Because of larger radii, the torque of subepicardial fibres dominates and accounts for the normal ‘positive’ counter-clockwise rotation of the LV apex. Thus, in the early stages, the loss of the inner subendocardial fibres owing to ischemia or fibrosis, and accounting for the reduced longitudinal function, will also allow the outer subepicardial fibres, which rotate in an opposite direction, to have increased leverage over the ventricle so, paradoxically, observed rotation may increase.24 Previous studies have found that, in LV hypertrophy, apical twist and untwist might be augmented or reduced.25, 26 Similarly, torsion is increased in patients with mild diastolic dysfunction but reduced in those with more severe degrees of diastolic dysfunction.27 The LV torsion and the subsequent rapid untwist appear to be manifestations of elastic recoil thus linking systolic contraction to suction and early diastolic filling, and increased suction is essential to produce rapid filling during exercise.28 Another explanation for the discordance between lengthening (longitudinal function) and twist is torsional dyssynchrony. Tan et al.29 found that in HFNEF untwist occurs before longitudinal lengthening and both untwist and longitudinal displacement were delayed particularly on exercise, unlike the coupled and nearly simultaneous longitudinal and untwist motion observed in control subjects.
Chamber contractility was also evaluated in this study. LVEF is recognized to be a poor measure of contractility because of its sensitivity to load and chamber remodelling.22 To accurately assess contractility, preload and afterload must both be accounted for. Using load-independent measurements (PRSWI and Ees), we observed that LV contractility was impaired at rest and deteriorated during exercise more in HFNEF than in controls. Therefore, a normal LVEF does not imply normal LV function. Furthermore, myocardial contractile reserve responses with exercise were blunted in HFNEF. The mechanisms limiting contractile reserve in HFNEF remain undefined. While one previous study reported that contractility in HFNEF is comparable to control at rest,7 a recent population-based study revealed that chamber contractility is significantly impaired in HFNEF.23 Abnormalities in calcium handling and energy substrate bioavailability, which have been demonstrated in HFNEF, may contribute.30
Peripheral mechanisms in HFNEF patients during exercise
This study revealed impairment of HR reserve, a non-diastolic peripheral factor, in HFNEF during exercise. We demonstrated that peak HR during exercise and its reserve were significantly lower in HFNEF than controls. Several investigators have also reported that HFNEF have attenuated HR response to exercise, similar to HFREF. Furthermore, those with such chronotropic incompetence have worse exercise intolerance than those without.3 Changes in HR with exertion are related to the withdrawal and reactivation of vagal tone. Accordingly, impairment in chronotropic reserve suggests that HFNEF patients may have abnormal autonomic function. With regard to the indices of arterial resistance derived, such as Ea and TAC, we did not find significant differences among subgroups, which were inconsistent with previous data.5 As the mechanisms of vascular accommodation during exercise in HFNEF remain speculative, a larger-scale study is warranted to investigate these issues further.
Limitations of the study
The echocardiographic images were acquired in different levels of exercise according to the study protocol, which may produce bias in our study. Further, echocardiographic and its derived parameters have inherently greater variability than invasive methods, which may influence the results. Moreover, we were unable to provide the results for biomarkers (e.g. BNP or NT-proBNP) because of some uncontrollable factors in the management of blood tests in our hospital.
Conclusions
Abnormalities of ventricular and peripheral dysfunction were evident in HFNEF at rest and there was further deterioration during exercise, including impaired LV global multidirectional strain, decreased LV long axis function and blunted chronotropic response to exercise. The finding that HFNEF had a lesser degree of impaired long axis augmentation at stress than HFREF warrants further clinical studies.
Acknowledgements
We extend our heartfelt thanks to Professor Cheuk-Man Yu and Professor Bryan Yan for their support of this study.
Funding statement
This research was funded by General Research Fund (project #479509) from the Research Grants Council of Hong Kong.
Conflict of interest: none declared




