Invasive Breast Cancer after Mastectomy and Autologous Breast Reconstruction
Abstract
Recurrence or new breast cancer after mastectomy and autologous breast reconstruction is a rare occurrence. We present a case series of four patients with this rare phenomenon and describe their presentation, workup, and surgical and adjuvant therapy. We discuss the possible physiologic mechanisms leading to tumor recurrence in a reconstructed breast along with literature review. A new palpable mass in a reconstructed breast mound warrants tissue diagnosis to avoid delay in treatment. The surgical options for breast cancer in tissue reconstructions depend on the location and extent of the tumor, involvement of the vascular pedicle, stage of disease, and adjuvant therapy options.
1. Introduction
Mastectomy and immediate breast reconstruction (IBR) have been shown to be oncologically safe for women with early stage breast cancer [1, 2]. Options for IBR include tissue expander placement and autologous flaps. Recurrence or the development of a new primary breast cancer after mastectomy and autologous breast reconstruction is rare. We present four patients with this unusual phenomenon and discuss their surgical management, adjuvant treatment, and outcomes.
2. Case Presentations
2.1. Case A
A 53-year-old woman underwent left skin-sparing mastectomy (SSM) with immediate free transverse rectus abdominis myocutaneous (TRAM) flap reconstruction with anastomosis to internal mammary vessels for a 1.9 cm area of ductal carcinoma in situ (DCIS), intermediate nuclear grade, estrogen (ER), and progesterone receptor (PR) positive.
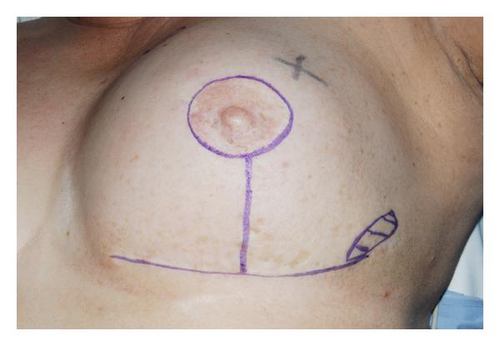
Six years later, she noticed a mass in the medial aspect of the same breast. Examination revealed two masses, 2 cm each, located in the upper inner and lower outer quadrants of the reconstructed breast mound (Figure 1). Diagnostic mammography revealed an area of increased density within the central portion of the reconstructed breast and four satellite nodular densities. Ultrasound revealed an irregular 1.4 cm mass in the central breast and a 1.5 cm mass in the upper inner quadrant. Core needle biopsy of these lesions showed moderately differentiated invasive ductal carcinoma (IDC). An 18FDG-PET scan revealed four foci of increased metabolic activity within the left breast and one in the left axilla. Breast MRI revealed enlarged axillary lymph nodes and five contrast-enhancing masses suspicious for malignancy in the left breast, the largest being a 4.0 cm mass invading the TRAM flap pedicle (Figure 2).

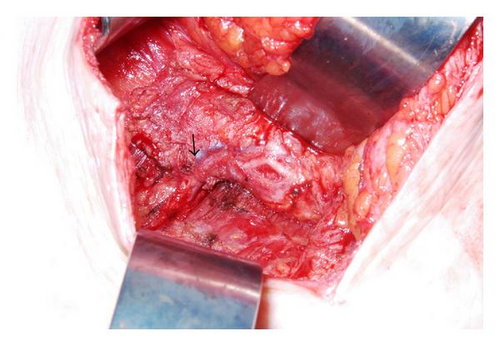
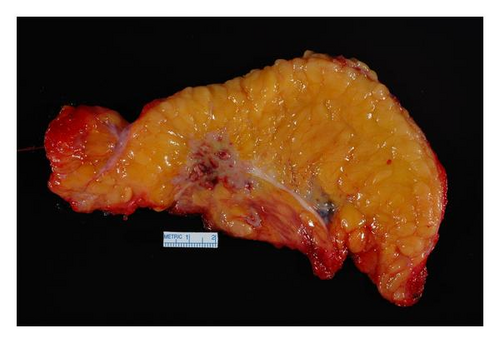
SSM with excision of the entire TRAM flap, partial excision of underlying pectoralis muscle, and complete axillary lymph node dissection were performed with immediate subpectoral tissue expander placement with AllodermR (Figure 3). Five foci of poorly differentiated IDC, ER/PR positive, Her2/neu negative, were identified; the largest focus measured 4.7 cm (Figure 4). No angiolymphatic invasion was seen. The malignant glands were present within adipose tissue containing adnexal structures consistent with subcutaneous tissue. No residual breast tissue was identified histologically. One of twenty-six axillary lymph nodes was positive for metastatic carcinoma without extranodal extension.
She received adriamycin, cyclophosphamide, and paclitaxel chemotherapy, chest wall radiation, and hormonal therapy and is doing well at 6 months of follow-up.
2.2. Case B
A 44-year-old woman underwent right breast wide local excision for poorly differentiated multifocal IDC, basal subtype (triple negative), the largest focus measuring 2.3 cm. One sentinel lymph node was removed, which was benign. She received adjuvant chemotherapy (adriamycin, cyclophosphamide, and paclitaxel). Eight months later, she was diagnosed with local recurrence in the ipsilateral breast and underwent a right SSM and contralateral (left) risk reduction SSM followed by immediate bilateral deep inferior epigastric perforator (DIEP) flap reconstruction. Pathology revealed a 0.8 cm poorly differentiated IDC, basal subtype (triple negative), with two negative sentinel lymph nodes.
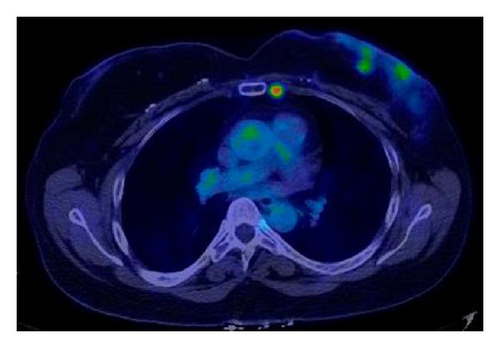
Five years later, she noted a lump in the lower outer quadrant of the contralateral reconstructed left breast (risk reduction side). Breast MRI revealed a 4.5 cm irregular mass within the left breast mound. The patient expressed a strong desire for preservation of the breast mound, and therefore, wide local excision of the DIEP flap in the area of the tumor was performed with concurrent left sentinel lymph node biopsy. Pathology revealed a 5.0 cm poorly differentiated IDC, basal subtype, with angiolymphatic invasion. Two axillary sentinel lymph nodes were negative for metastasis. No residual breast tissue was identified in the resected specimen. A staging 18FDG-PET scan (Figure 5) performed postoperatively revealed two foci of intense uptake within the left breast suspicious for residual cancer and a hypermetabolic internal mammary lymph node.
She then received chemotherapy consisting of adriamycin and cyclophosphamide followed by paclitaxel. The internal mammary lymph node resolved on chemotherapy; however, the two breast masses progressed, necessitating a complete takedown of the DIEP flap five months later. Pathology revealed four foci of poorly differentiated IDC, ranging in size from 0.4 to 1.4 cm. She completed (6000cGy) radiation to the left chest wall including the left supraclavicular region and the internal mammary nodal chain.
2.3. Case C
A 62-year-old woman was treated with wide local excision for a 0.8 cm moderately differentiated ER/PR positive IDC of the right breast. One sentinel lymph node was negative for metastatic carcinoma. She declined adjuvant radiation and presented three years later with a mass in the ipsilateral breast which proved to be IDC on core biopsy. She underwent total mastectomy and pathology revealed a 0.8 cm moderately differentiated, ER/PR positive, Her2/neu negative IDC with a single negative lymph node. No lymphovascular invasion was identified. She received adjuvant anastrozole and underwent delayed DIEP flap reconstruction of the ipsilateral breast six months later.
One year later after her reconstruction, she presented with a mass in the scar from her original primary tumor, which proved to be carcinoma on core biopsy. Wide local excision of the DIEP flap in this area down to and including the pectoral muscle revealed a 1.4 cm moderately differentiated ER/PR positive, Her2/neu negative IDC confined to the skin. No residual breast tissue was identified in the resected flap. She has completed radiation to the reconstructed breast and declined systemic therapy.
2.4. Case D
A 45-year-old woman underwent skin-sparing modified radical mastectomy for 0.7 cm, well differentiated IDC associated with a surrounding 4 cm area of DCIS. Four of twenty-seven axillary lymph nodes were positive for metastatic carcinoma. Immediate reconstruction was performed using free TRAM flap. She then received adjuvant chemotherapy (adriamycin, cyclophosphamide followed by paclitaxel) and hormonal therapy (Aromasin).
Routine examination three years later revealed a mass over the lateral aspect of the TRAM flap. Diagnostic mammogram revealed a focus of microcalcifications, which were malignant on core biopsy. Wide local excision of the TRAM flap was performed. Pathology revealed a 0.5 cm moderately differentiated ER positive, PR negative, Her2/neu negative IDC associated with extensive DCIS. There was no lymphovascular invasion, and no residual breast tissue was identified. She is completing radiation to the breast and supraclavicular area and is taking Aromasin.
3. Discussion
We have presented four diverse cases of breast carcinoma recurrence after autologous tissue reconstruction (Table 1), which highlight the challenges and management options for these patients. Our report also illustrates how implant-based breast reconstruction can be used when the entire tissue flap requires removal.
| Case | Initial tumor | Initial reconstruction | Recurrence | Surgical treatment of recurrence | Adjuvant treatment | Outcome |
|---|---|---|---|---|---|---|
| A | DCIS | SSM with immediate free TRAM flap | IDC | SSM, complete TRAM excision, implant-based reconstruction | Chemotherapy, hormonal therapy and local radiation | Alive without evidence of disease |
| B | None (Prophylactic mastectomy) | SSM with immediate DIEP flap | IDC | Initial WLE followed by complete DIEP excision | Chemotherapy, locoregional radiation | Alive with recurrence |
| C | IDC (0.8 cm) | SSM with Delayed DIEP flap | IDC | WLE | Local radiation | Alive without evidence of disease |
| D | IDC (0.7 cm) | SSM with immediate free TRAM flap | IDC | WLE | Locoregional radiation and hormonal therapy | Alive without evidence of disease |
Women undergoing skin-sparing mastectomies with immediate breast reconstruction (IBR) for small tumors (T1 or T2) have recurrence rates similar to those patients with delayed or no reconstruction [1–3]. The overall incidence of locoregional recurrence after mastectomy ranges from 2–15% at five years [4–6]. Tissue transfer procedures are an oncologically safe option for breast reconstruction [7, 8]. A 4–6% prevalence of skin flap or chest wall recurrences following autologous breast reconstruction using TRAM flaps has been reported [3, 9] and regional nodal recurrences occur in up to 15% of patients after TRAM flap reconstruction [10, 11].
Limited assessment of the factors associated with local recurrences after SSM and IBR exist. In a study by Kroll et al. [4] local recurrence (LR) rates were lower in patients with T1 tumors compared to T2 tumors (6% versus 9%), but this difference was not statistically significant. A trend was also identified for a high nuclear grade being associated with a higher risk of LR. Another study of 173 patients by Medina-Franco et al. [6], in which the majority of patients (92%) had tissue reconstruction, identified tumor stage II or III, tumor size greater than 2.0 cm, node positivity and poor tumor differentiation to be associated with increased LR. Local recurrence was an independent predictor of reduced survival after SSM and IBR, as were primary tumor stage, poor tumor differentiation, and absence of treatment with hormone therapy. In this study, 75% of patients who developed LR within a median follow-up of 73 months developed distant metastases and died of disease within a mean of 21 months. Other reports of patients with recurrent breast cancer after mastectomy have shown that approximately 70% of LRs occur in the first 36 months [12, 13], while 90% are detected within five years of initial diagnosis [14]. Although several studies have reported worse prognosis with LR after mastectomy than after breast conservation therapy [8, 14–16], this more adverse impact of LR on survival after mastectomy has not been supported by others [3]. In a study by Gilliland et al. all patients with LR developed distant metastases between 1.2 and 4.2 years and all died of disease between 2.5 and 7.2 years [14]. Other reports with longer term follow-up of up to 10 years among patients with LR after mastectomy have reported overall survival rates of 18–42% [14, 17] and 10-year disease-free survival of 7–17% after the diagnosis of LR [15, 16]. In contrast, the MD Anderson experience of 23 patients with LR after SSM and IBR revealed 61% of patients alive with no evidence of disease at a median follow-up of 26 months [3]. However, survival after breast cancer has been consistently shown to be associated with stage and grade of the primary tumor [6, 14, 17, 18], and therefore, distant relapse seen synchronously with LR can mostly be attributed to tumor biology rather than type of surgical treatment performed. However, not all local recurrences are equal. Patients with subcutaneous tissue recurrences have a better survival, decreased incidence of distant metastases, and greater chance of remaining disease free than those with true chest wall recurrences [19].
The possible mechanisms of development of LR or a new cancer after mastectomy and IBR are not well elucidated. Possible explanations include residual cancer, benign residual breast tissue progressing to cancer, tumor spillage at the time of the initial operation, and hematogenous tumor spread. All forms of mastectomy, whether radical, modified radical, or skin-sparing, leave some residual breast tissue. Concerns have been raised about the increased residual breast tissue remaining after SSM because of the length of the skin flaps and retention of the inframammary fold. Carlson et al. used computer image analysis to examine the inframammary fold tissue retained with a SSM [20]. Residual breast tissue was identified in 13 of 24 specimens but was correlated to only 0.02% of the total tissue volume removed. Barton et al. demonstrated equivalent amounts of residual glandular breast tissue remaining on the anterior chest walls of 27 patients who had undergone SSM compared to 28 patients following total mastectomy, based on multiple chest wall biopsies [21]. The fact that histologic examination of LR rarely shows identifiable breast tissue has been highlighted by others [20]. Some researchers have proposed that skin and subcutaneous recurrences may be a result of residual tumor cells that were separate from an adequately resected cancer [19]. Displacement of tumor cells can occur after core needle biopsies of breast tumors [22]. Although it is not conclusive that displacement of tumor cells leads to recurrence, it is recommended that needle biopsy exit sites be excised at the time of definitive surgery. However, incorporating these biopsy sites in a SSM incision can be technically challenging. Reassuringly though, despite changes in surgical approaches over time, the LR rate after total mastectomy for breast cancer has remained relatively constant. It is, therefore, plausible that factors found to be associated with LRs, that is, stage and grade of primary tumor, also dictate distant failures and prognosis.
The issue of routine screening of the reconstructed breast is unsettled and routine mammography is not generally recommended. However, some researchers recommend routine mammograms after SSM and IBR in patients initially presenting with multifocal or multicentric primary tumors [23]. Most recurrences after SSM and IBR are superficial subcutaneous nodules, which are easily appreciable on physical examination.
However mammography should be performed after a recurrence is suspected, as well as a breast ultrasonography if indicated [3]. MRI can discern between benign and malignant findings in patients with palpable abnormalities or pain after TRAM flap reconstruction [24]. Some researchers recommend patients undergoing TRAM flap reconstruction to be followed with a baseline MRI within six months after the surgical procedure and annually thereafter. If the patient has a BI-RADS category 3 lesion in the TRAM flap or contralateral breast, follow-up imaging at four to six months is recommended. For patients with BI-RADS category 4 or 5 lesions in the TRAM flap or contralateral breast, a biopsy is indicated [25]. The role of vigilant and regular physical examination cannot be over emphasized. All patients undergoing mastectomy, including prophylactic mastectomy, should be counseled of the continued risk of breast cancer after mastectomy.
A new palpable mass in a reconstructed breast mound warrants imaging evaluation and, where appropriate, a tissue diagnosis, preferably with a core biopsy to avoid a delay in treatment. All four of our patients had subcutaneous disease, a more common presentation than true chest wall recurrences. Case A had pectoralis invasion from the bulky subcutaneous cancer that was not diagnosed early.
The surgical options for breast cancer in patients with tissue reconstruction depend on the location and extent of the tumor, involvement of the vascular pedicle of the flap, stage of disease, and adjuvant therapy options. An evaluation for distant metastatic disease should be undertaken which should include complete blood count, liver function tests, CT scan of abdomen and pelvis as well as a bone scan. In cases of LR only, excellent disease control without sacrificing the reconstructed breast mound can be achieved in most patients. The goal of surgical treatment is to achieve clear resection margins. However, excision of a TRAM flap should be strongly considered in patients with multifocal or multicentric recurrences. This treatment is necessary if the recurrence involves the TRAM flap pedicle. Excision alone for patients with LR after mastectomy is associated with failure rates of up to 75% [26]. Adjuvant radiation therapy to the residual TRAM flap or the chest wall is recommended. Adjuvant systemic therapy should also be considered for these patients as LR is a manifestation of aggressive primary disease and these patients are at significant risk for systemic failure.
Nodal evaluation in patients with LR after mastectomy with reconstruction is dependent on individual patient factors. The accuracy of sentinel lymph node (SLN) surgery in these patients is unknown. Reports of SLN in patients with recurrent breast cancer have included a few case reports of successful lymphatic mapping after mastectomy [27, 28]. Currently SLN surgery in this setting has not been validated and therefore should only be performed with concomitant axillary lymph node dissection. In patients where the axilla has already been dissected, SLN mapping may be helpful to identify residual nodal tissue in the axilla or extraaxillary sites at risk for metastases. The use of lymphoscintigraphy is especially important in these situations. Sentinel node mapping in our patients showed localization to the ipsilateral axilla, despite prior mastectomy and TRAM reconstruction. The importance of axillary staging in LR is questionable. Historically, the majority of these patients had undergone complete axillary lymph node dissection at the time of initial surgical resection of the primary breast tumor. In the current age of SLN surgery, we are encountering patients with recurrent breast cancer with residual axillary lymph nodes. The majority of patients with LR receive chemotherapy and radiation regardless of the nodal evaluation and a multidisciplinary evaluation of these patients is important.
Local recurrence after mastectomy and autologous tissue reconstruction is fortunately a rare phenomenon. Any new subcutaneous mass warrants evaluation and a biopsy if the workup is suspicious for recurrent cancer. Local control rates and patient survival are high, even after a diagnosis of LR especially in low risk patients with early stage breast cancer. These results can often be achieved while maintaining most of the reconstructed breast mound.
Abbreviations
-
- DCIS:
-
- Ductal carcinoma in situ
-
- SSM:
-
- Skin-sparing mastectomy
-
- TRAM:
-
- Tranverse rectus abdominis myocutaneous
-
- DIEP:
-
- Deep inferior epigastric perforator
-
- WLE:
-
- Wide local excision
-
- IDC:
-
- Invasive ductal carcinoma




