Epididymosome-Mediated Acquisition of MMSDH, an Androgen-Dependent and Developmentally Regulated Epididymal Sperm Protein
Funded by the Department of Science and Technology, Government of India, a Senior Research Fellowship from the ICMR (A.R.S.), and a Junior Research Fellowship from the Department of Science and Technology (C.S.J.).
Abstract
Abstract: A differential proteomics approach led to the identification of several novel epididymal sperm proteins. One of the novel proteins was methylmalonate-semialdehyde dehydrogenase (MMSDH). In the present study, we carried out an in-depth characterization to study its regulation by androgen, its appearance during ontogeny, and the mechanism of its interaction with and acquisition by the sperm. Western blotting and immunohistochemical studies suggest that the protein is present in both tissue and sperm from all regions of the epididymis, indicating synthesis as well as acquisition of the protein in these regions. Androgen depletion resulted in reduction of the MMSDH protein level in the epididymis, which completely disappeared 1 week after castration. The protein reappeared after testosterone propionate injection, indicating that the protein is regulated by androgens. Ontogeny studies indicated that the protein appeared from day 10 postnatal with a gradual increase at day 30, which maximized at day 50, indicating that the protein is developmentally regulated and is probably involved in epididymal development. Sequential extraction of sperm proteins indicated that MMSDH exists both as a peripheral and integral form on the plasma membrane. We also found that the protein can be transferred from the epididymosomes to testicular sperm in vitro. The study provides evidence regarding the acquisition of this multidomain androgen and developmentally regulated protein in the epididymis via the epididymosomes. The molecule has generated enough interest and deserves to be investigated further for its physiological relevance.
Highly differentiated testicular sperm are immature and lack fertilizing ability. These sperm transit through a convoluted tubule, the epididymis, which is anatomically divided into the initial segment, the caput, the corpus, and the cauda regions (Orgebin-Crist, 1969; Jervis and Robaire, 2001). The epididymis is involved in sperm transport, concentration, storage, and, more importantly, sperm maturation. Sperm maturation involves multifaceted alterations in the protein composition of the sperm plasma membrane that lead to the attainment of vital functions such as forward motility and fertilizing ability (Cuasnicu et al, 1984a; Cooper and Yeung, 2006). The maturational events are brought about either by uptake of new proteins synthesized and secreted by the epithelial cells of the epididymis or posttranslational modifications of existing proteins on the sperm or relocalization of existing proteins. The luminal microenvironment created by the secretions of the epithelial cells of the epididymis provides the required environment for the maturational changes (Jones, 1998).
It has been shown that the changes in morphology and function of the developing epididymis are under the control of androgens and estrogens and are accompanied by the presence of proteins such as androgen receptor and estrogen receptor, occludins, and cadherins (Rodriguez et al, 2002). Among epididymis-specific genes, the HE1 and beta-galactosidase—like gene (Glb1l4) mRNA is up-regulated distinctly at puberty until adulthood (Uhlenbruck et al, 1993), with the latter reported to be maximally expressed at puberty and involved in epididymal cell differentiation as well as regional development (Zhen et al, 2009). The epididymis is an androgen-regulated tissue wherein testosterone (T) and its metabolites, dihydrotestosterone and estradiol, are established as the principal regulators of epididymal structure and functions, with T playing the key role. Orchiectomy eliminates both circulating androgens and testicular factors and results in the decrease or disappearance of some of the epididymal protein, whereas supplementation of androgens reverses these effects, resulting in the reinstatement of some of the proteins (Robaire et al, 2007).
Under androgenic control, proteins synthesized by cells of the epididymal epithelium are secreted in the lumen (Cuasnicu et al, 1984b; Robaire and Viger, 1995) and transported to the sperm to make them a fully functional gamete (Sullivan, 1999; Cuasnicu et al, 2002; Dacheux and Dacheux, 2002). Proteins secreted in the lumen follow either a classical (ie, merocrine) or nonclassical (ie, apocrine) mode of secretion (Sullivan et al, 2007). Many of the epididymal proteins are referred to as coating proteins, which bind to the sperm membrane through electrostatic interaction and therefore could be washed away with a high—ionic strength solution. However, a few epididymal proteins are integral in nature and cannot be easily removed (Cooper, 1998). Most secretory proteins of eukaryotes are synthesized as precursor polypeptides containing a cleavable N-terminal signal. A class of secretory proteins do not possess hydrophobic signal sequences and leave the cell by a nonclassical route of apocrine secretion. Apocrine secretion is brought about by blebs and protrusions at the apical surface of secretory epithelial cells very often seen in the male reproductive system (Manin et al, 1995). These blebs detach and are shed into the intraluminal compartment. They disintegrate, and the small membranous residues known as epididymosomes are released (Aumuller et al, 1997, 1999; Hermo and Jacks, 2002; Hermo and Robaire, 2002). Several studies have revealed that these vesicles, present in the cauda epididymis and seminal plasma, transport a number of proteins to sperm, some of which have been reported to be involved in sperm motility and fertility (Legare et al, 1999; Frenette and Sullivan, 2001; Frenette et al, 2002, 2003, 2005, 2006; Rejraji et al, 2002; Sullivan et al, 2005).
With the advancement of “omics” research, new epididymal molecules have been identified by several investigators (Chaurand et al, 2003; Baker et al, 2005, 2008a, 2008b; Cao et al, 2006; Martinez-Heredia et al, 2006; Yuan et al, 2006; Aitken et al, 2007; Khan et al, 2009; Sutovsky, 2009; Moura et al, 2010; Suryawanshi et al, 2011); however, their functional potential is yet to be determined. Using differential proteomics, we reported a novel protein, methylmalonate-semialdehyde dehydrogenase (MMSDH; accession Q02253), also known as aldehyde dehydrogenase family 6 member A1 (ALDH6A). We validated MMSDH for its epididymal specificity, conserved nature, and surface localization (Suryawanshi et al, 2011). The present study was carried out to gain an insight into the importance of this molecule in sperm function. Toward this end, we investigated its regulation by androgens and its appearance during ontogeny. We further analyzed its synthesis by the epididymis and mechanism of its interaction and acquisition by the sperm.
Materials and Methods
Materials
Nitrocellulose membranes (Hybond-C Extra) and ECL Plus Western blotting detection reagents were obtained from GE Health Care (Buckinghamshire, United Kingdom). Monoclonal antibodies to β-actin were obtained from Sigma (St Louis, Missouri). Protein molecular weight markers from Fermentas (Glen Burnie, Maryland), chemicals for preparation of buffers, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), Western blotting, and Dulbecco Modified Eagle Medium were procured from Qualigens (Mumbai, India), SRL India Ltd (Mumbai, India), and Hi—media (Mumbai, India). Restore Western blot stripping buffer was procured from Pierce (Rockford, Illinois). Polyclonal antibody to ALDH6A protein (MMSDH) was procured from Abcam (Cambridge, United Kingdom) and secondary antibody from Dako Cytomation (Glostrup, Denmark).
In Silico Analysis of MMSDH
The amino acid sequence of MMSDH (accession Q02253) was extracted from the Uniprot Knowledgebase (http:www.uniprot.orguniprotQ02253). A motif search of MMSDH was carried out by SSDB Motif search (http:www.kegg.jpssdb-binssdb_motifkidrno:81708). The SignalP 3.0 server online tool (http:www.cbs.dtu.dkservicesSignalP) was used to predict the presence and location of signal peptide cleavage sites in amino acid sequences, and the Secretome P2.0 Server online tool (http:www.cbs.dtu.dkservicesSecretomeP) was used to predict the nonclassical secretory proteins.
Animals
Adult inbred male Holtzman (HM) rats weighing 180–220 g used in the study were maintained at a temperature of 22°C—23°C, humidity of 50%–55%, and a light-dark cycle of 14 hours light and 10 hours dark, with food and water available ad libitum. Inbred male HM rats 5, 10, 20, 30, 40, 50, and 60 days of age were used for ontogeny studies. The experimental protocols for use of animals were approved by the Institutional Ethics Committee for Care and Use of Laboratory Animals for Biomedical Research.
Collection of Tissues and Sperm
Adult male rats were sacrificed to remove testes; epididymides; somatic tissues such as liver, kidney, lungs, spleen, and thymus; and seminal vesicle, prostate, and vas deferens male reproductive tissues. The samples were placed separately in 0.1 M phosphate-buffered saline (PBS), pH 7.4. Initial segment, caput, corpus, and cauda epididymal regions were separated from whole epididymides. Testis and all the regions of epididymides were teased separately in PBS and incubated at 37°C for 30 minutes. The supernatant containing sperm were collected in separate tubes, and the tissue pellets were washed at least 5 times with 0.1 M PBS to remove the sperm from tissues completely. The supernatant containing sperm was spun down at 500 × g for 20 minutes at 4°C to get the sperm pellet. This pellet was washed thrice with PBS at 500 × g for 20 minutes. The sperm and tissue pellet from testis, all the regions of epididymis, and other tissues were used for protein extraction.
Castration and Androgen Replacement
The procedure was performed as described previously (Zhu et al, 2007) with a minor modification. Adult normal male HM rats were castrated bilaterally after sodium pentobarbital anesthesia. Animals were divided into 10 groups (3 rats per group) and sacrificed on days 0, 1, 3, 5, 7, and 10 after castration, as well as 1, 3, 5, and 7 days after androgen supplementation, which was started on the 10th day after castration. Rats were injected with testosterone propionate (3 mg/kg body weight) every day. The epididymides were excised and weighed and total proteins were extracted. Serum samples for each group were collected for the measurement of testosterone concentration by enzyme-linked immunosorbent assay using a total testosterone assay kit as per the instructions of the manufacturer.
Postnatal Development
Normal male rats aged 5, 10, 20, 30, 40, 50, and 60 days (n = 5) were anesthetized by sodium pentobarbital and sacrificed. Epididymides were removed and placed in 0.1 M PBS solution. Epididymal tissues obtained from each postnatal age were used to analyze samples in triplicate experiments.
Protein Extracts and Western Blot Analysis
Proteins were extracted as per the procedure described previously (Suryawanshi et al, 2011). Briefly, the tissues and sperm pellets were teased and incubated overnight with radioimmunoprecipitation buffer containing 50 mM Tris HCl, pH 8, 150 mM NaCl, 1% NP-40, and 0.25% sodium deoxycholate. On the following day, the suspension was sonicated at 4°C (Ralsonics Ultrasonic Processor, Model RP-120–122, Mumbai, India) at 100% output with 1-minute bursts at 30-second intervals for a total of 5 minutes. The sonicated sample was then centrifuged at 10 000 × g at 4°C for 10 minutes. Protein in the supernatant was quantitated by Bradford assay. Each protein sample (20 μg) was separated on 10% SDS-PAGE and blotted on nitrocellulose membranes (Hybond-C Extra; GE Healthcare Biosciences, Kowloon, Hong Kong). The polyclonal antiserum against rat ALDH6A (MMSDH) protein was used as the primary antibody (dilution 1:25). The second antibody was a swine horseradish peroxidase—conjugated anti-rabbit immunoglobulin G (IgG; dilution 1:3500; Dako Cytomation). The peroxidase activity was demonstrated with a chemiluminescent substrate (ECL Plus Western blotting detection reagents; GE Healthcare). To ascertain that the protein load of sperm/tissue protein was equal in all the lanes, the same membranes were stripped with the Restore Western blot stripping buffer and reprobed using a mouse monoclonal anti–β-actin antibody (Sigma). Each Western blot experiment was performed in triplicate.
Immunohistochemical Localization
Rat epididymis and testis were fixed in Bouin fixative for 24–48 hours. The tissues were processed and embedded in paraffin wax using automated tissue processor model ASP 200 from Leica Biosystems (Nussloch, Germany), and serial sections of 5 μm were cut and placed onto clean poly-l-lysine—coated glass slides. Immunohistochemical localization was done according to the manufacture's instructions (Vectastain Laboratories, Peterborough, United Kingdom). Briefly, slides containing 5-μm sections were deparaffinized. The slides were subsequently hydrated through alcohol grades; deparaffinized sections were treated with 0.3% (vol/vol) H2O2 in methanol for 30 minutes to block endogenous peroxidase activity. Antigen retrieval was performed by placing slides in a container with citrate buffer containing 10 mM citric acid, 0.05% Tween 20, pH 6.0, and microwaving for 5 minutes. After slides cooled to room temperature (RT), 3 washes with PBS were given to remove salt deposits. To block nonspecific binding, slides were incubated with blocking solution containing 5% (wt/vol) fish gelatin in PBS and 1.5% normal goat sera for 1 hour at RT. Slides were then incubated with rabbit polyclonal antibody to MMSDH (1:10) overnight at 4°C in a humidified chamber. Slides were washed 4 times with PBS and then incubated for 1 hour at RT with biotin conjugated anti-rabbit diluted 1:200 in blocking solution. The slides were then washed as described earlier. Slides were incubated with AB reagent for 30 minutes at RT. After 4 washes as described earlier, the color reaction was carried out with 3,3-diaminobenzidine (DAB) with 0.03% (vol/vol) H2O2 (10 mg DAB + 10 μLH2O2 in 10 mL PBS). The reaction was terminated with distilled water; slides were then counterstained with hematoxylin. After dehydration, the slides were mounted in DPX mountant (Sigma). For the control set, also referred to as the secondary-alone control, sections were incubated with buffer instead of serum.
Sequential Extraction of Sperm Proteins
To determine the mode of MMSDH binding to the sperm plasma membrane, sequential extractions were carried out according to the protocol described by Syntin and Cornwall (1999) with some modification. Briefly, epididymal sperm from cauda epididymis were isolated and washed 2 times with PBS to remove any loosely bound proteins. Sperm pellets were then sequentially extracted with increasing concentrations of sodium chloride (1, 2, and 4 M NaCl). Subsequently, sperm pellets were extracted with 0.1% Triton X-100 prepared in PBS. All extractions were carried out at RT for 20 minutes under constant mixing with 800 × gcentrifugations for 10 minutes after each treatment. Supernatants were dialyzed overnight against distilled water, lyophilized, and resuspended in Laemmli buffer. Sperm pellets after hypertonic salt solution as well as Triton X-100 treatment were divided into 2 parts. One part was heated with Laemmli buffer, and the other part was incubated with 1% SDS buffer at 4°C overnight, sonicated, and centrifuged at 10 000 × g, and the supernatant was collected. All samples were heat denatured at 100°C for 5 minutes in Laemmli buffer and subjected to SDS-PAGE according to Laemmli's (1970) protocol and transferred to a nitrocellulose membrane as per the protocol described by Towbin et al (1979). Immunoblotting was carried out under the protocol described by Suryawanshi et al (2011).
Isolation of Epididymosomes
Epididymosomes were collected as per the procedure described by Legare et al (1999). Briefly, epididymal fluid was collected by teasing tissues from the caput, corpus, and cauda segments in PBS. The suspension was centrifuged at 5000 × g for 20 minutes. The supernatant was recovered and resubjected to ultracentrifugation at 100 000 × g for 2 hours at 4°C. The pellet was resuspended in Laemmli buffer (100 μL) and processed for SDS Western blot analysis as described earlier.
Transfer of Protein from Epididymosomes to Testicular Spermatozoa
Protocol for protein transfer from epididymosomes to testicular sperm was followed as described by Frenette et al (2002). Briefly, testicular sperm at a concentration of 20 million sperm/mL were suspended in buffer A with ZnCl2 (0.15 M NaCl and 1 mM ZnCl2, pH 6.5) and buffer B without ZnCl2 (0.15 M NaCl, pH 6.5). To these, epididymosomes from cauda were added and incubated at 37°C for 3 hours. At the end of the incubation period, sperm were centrifuged at 1000 ×g for 10 minutes and then washed with 0.1 M PBS 4 times. The final pellet obtained was boiled in Laemmli buffer for 5 minutes and used for SDS-PAGE—Western blot analysis as described above. After incubation, cell death was assayed with 1% trypan blue stain.
Results
In Silico Analysis of MMSDH
In silico analysis of protein MMSDH (ALDH6A) is represented in Figure 1. The MMSDH sequence consists of 535 amino acid (aa) with transit peptide domain (1–32aa) and chain (33–535aa). MMSDH belongs to the aldehyde dehydrogenase family, which consists of Aldedh, acyl—coenzyme A (CoA) reductase (LuxC), and aldehyde dehydrogenases cysteine active site prosite motif. Based on gene ontology annotation, MMSDH is predicted to localize subcellularly in mitochondria involved in fatty acyl-CoA binding and plays a role in valine and pyrimidine metabolism. MMSDH is also predicted to acetylate protein. In this study, we used SignalP 3.0 server to predict the presence and location of signal peptide cleavage sites in amino acid sequences of MMSDH. As a result, SignalP 3.0 server predicted the absence of signal peptide and also predicted that MMSDH does not follow the classical secretory pathway. Furthermore, we used Secretome 2.0 server, which confirmed that MMSDH protein follows the nonclassical secretory pathway. prediction tools predicted that MMSDH has no potential glycosyl-phosphatidylinositol (GPI) anchoring site. The best score for a GPI anchoring site is at the 520aa position in the MMSDH protein sequence, which is not significant.
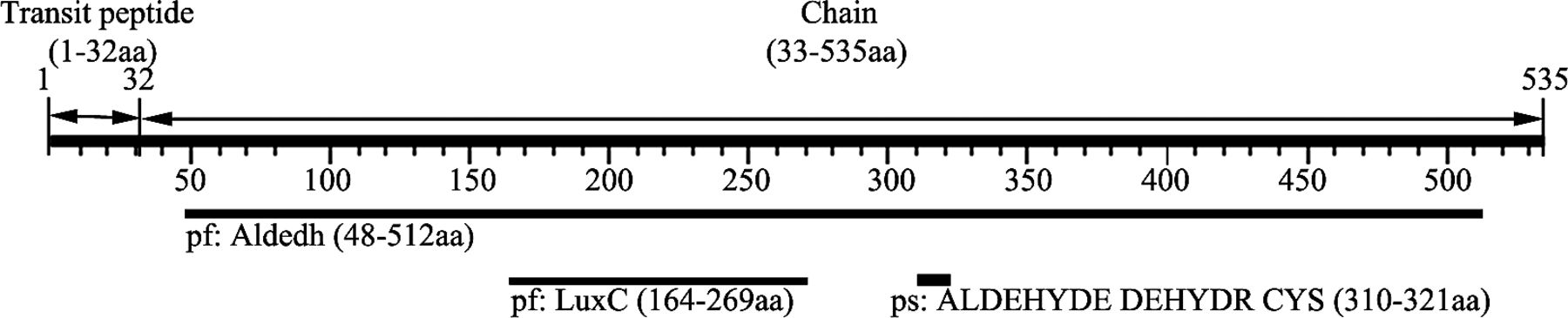
Computational analysis of methylmalonate-semialdehyde dehydrogenase (MMSDH). Sequence of MMSDH consists of 535 amino acid (aa), which consists of Aldedh, acyl—coenzyme A reductase (LuxC), and aldehyde dehydrogenases cysteine active site prosite motif. Amino acids 1–32 indicate the transit peptide domain, whereas 33–535aa represent the chain domain of the protein.
Regional and Tissue-Specific Expression
Western blot experiments were carried out to determine expression of MMSDH in sperm and tissues from testis and different regions of the epididymis, and the results are depicted in Figure 2. The protein was detected in sperm collected from the different regions of the epididymis initial segment, caput, corpus, and cauda (Figure 2A, lanes 2–5) but was not detected in the testicular sperm (Figure 2A, lane 1). The cognate protein at 57 kd was also detected in all the tissues of the epididymis (Figure 2B, lanes 2–5) but not observed in the testicular tissue (Figure 2B, lane 1). These data suggest that expression of the protein begins at the initial segment and remains throughout the length of the epididymal tubule. Blot probed with PBS or normal IgG, which served as negative control, did not show any reactivity (data not shown). To ensure equal load of protein in all the wells, blot was stripped with Restore stripping buffer and reprobed with a monoclonal antibody to β-actin, which revealed consistent load, as demonstrated in the lower panels of Figure 2.
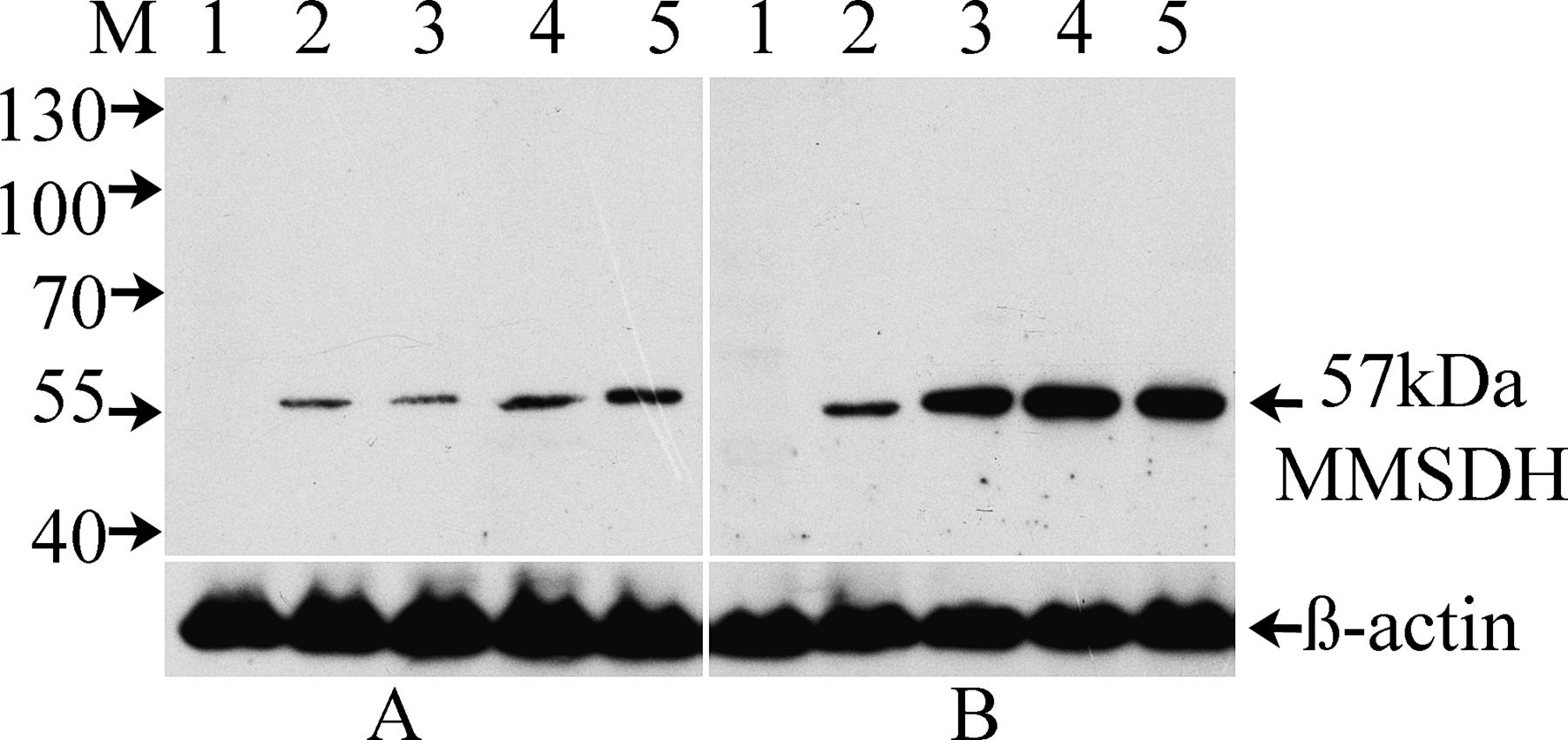
Western blot analysis showing region-specific expression of methylmalonate-semialdehyde dehydrogenase (MMSDH) in sperm and tissue collected from testis and epididymis. Blot was probed with rabbit polyclonal MMSDH antibody (1:25). (A) Sperm proteins from testis (lane 1) and the different regions of the epididymis, including the initial segment, caput, corpus, and cauda epididymis (lanes 2–5, respectively), and (B)their respective tissue proteins (lanes 1–5). The lower panel shows reactivity of the stripped blot with anti–β-actin antibody (1:5000).
The Western blot of the other tissues yielded reactivity only in liver, kidney, thymus, and seminal vesicle out of several tissues checked (data not shown).
Immunohistological Analysis
Immunohistochemical studies with rabbit polyclonal antibody to MMSDH were carried out on Bouin fixed testis and epididymal sections to gain further information about its localization pattern. Antibody showed specific immunoreactivity only in epididymis, as shown in Figure 3. There was no staining observed in the testicular section. Staining was observed in all regions of the epididymis from the initial segment to the caudal region (Figure 3A). Positive staining was observed in the microvilli, principal cell, and luminal sperm in the initial segment and caput sections, whereas the staining in the corpus and cauda was restricted to the principal cell in the epithelium and the luminal sperm (Figure 3A). The data imply principal cell specificity of MMSDH. No staining was observed in the testis and epididymis sections probed with normal rabbit IgG (Figure 3B).
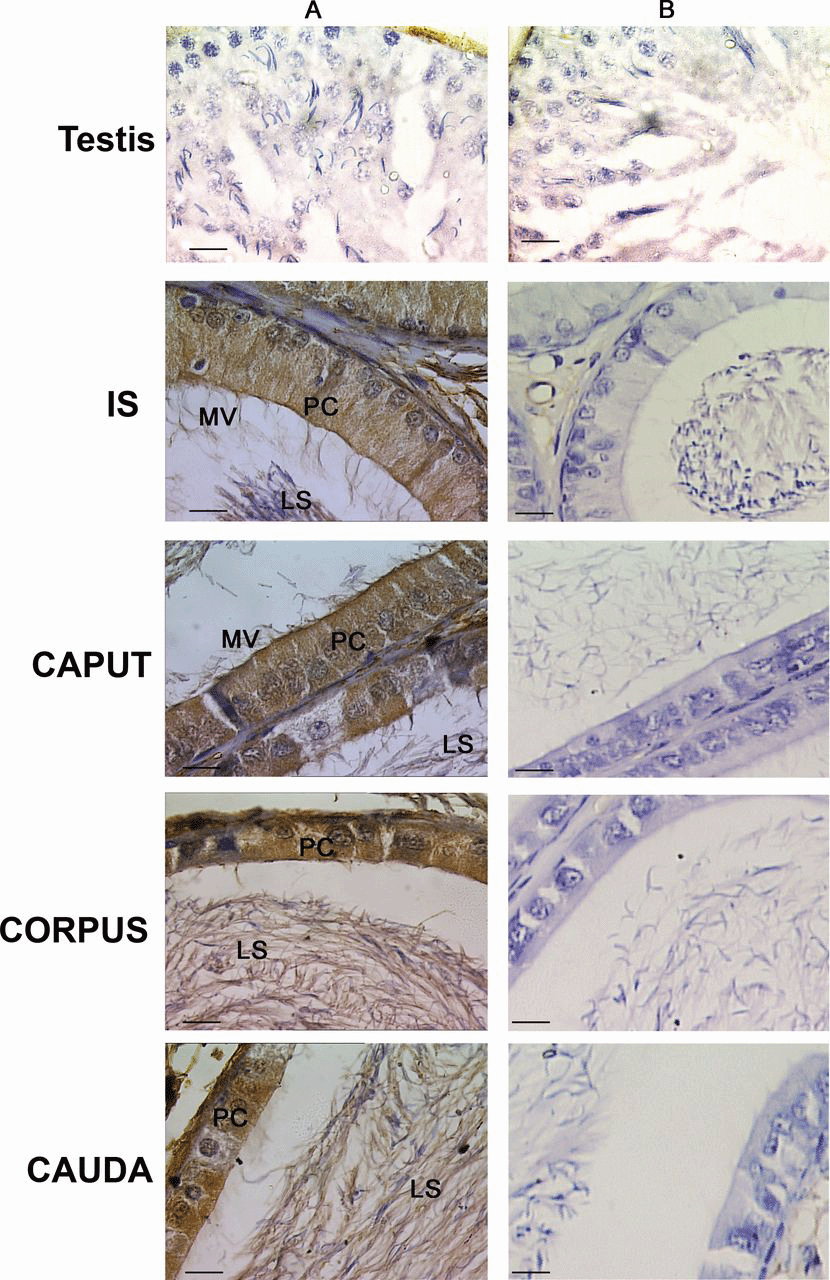
Immunohistochemical localization of methylmalonate-semialdehyde dehydrogenase (MMSDH) in testis and different regions of the epididymis, including the initial segment (IS), caput, corpus, and cauda epididymis. (A) Sections probed with the polyclonal MMSDH antibody (1:10). Positive staining was seen in the microvilli (MV), principal cell (PC), and luminal sperm (LS) in the IS and caput sections, whereas the staining in the corpus and cauda was restricted to the PC in the epithelium and the LS. (B)Sections incubated with normal rabbit immunoglobulin G, which served as the negative control, did not show any specific staining. Images were observed using light microscope, magnification × 100. Color figure available online at www.andrologyjournal.org.
Androgen Regulation
Because sperm maturation is an androgen-dependent process, we analyzed protein expression under conditions of androgen manipulation (Figure 4). Blot of whole epididymal proteins obtained from rats that were sham operated (day 10, lane 1), castrated (lanes 2–6), or castrated and supplemented (lanes 7–10) with testosterone propionate postcastration and probed with anti-MMSDH antibody. In the castrated animals, the serum testosterone level declined rapidly (data not shown). In parallel, an evident decrease was found in the MMSDH protein level on the first day postcastration (lane 2), which was nearly undetectable by the seventh day (lane 5), with complete loss of expression by the 10th day after surgery (lane 6). Testosterone replacement for the animals 10 days after castration resulted in a gradual increase in MMSDH protein level in the epididymis (lanes 7–10), suggesting that protein expression was regulated by testosterone. Blot stripping and reprobing with antibody to β-actin indicates equal load of proteins in all the lanes (lower panel). Buffer and normal IgG controls did not show any specific reactivity (data not shown).
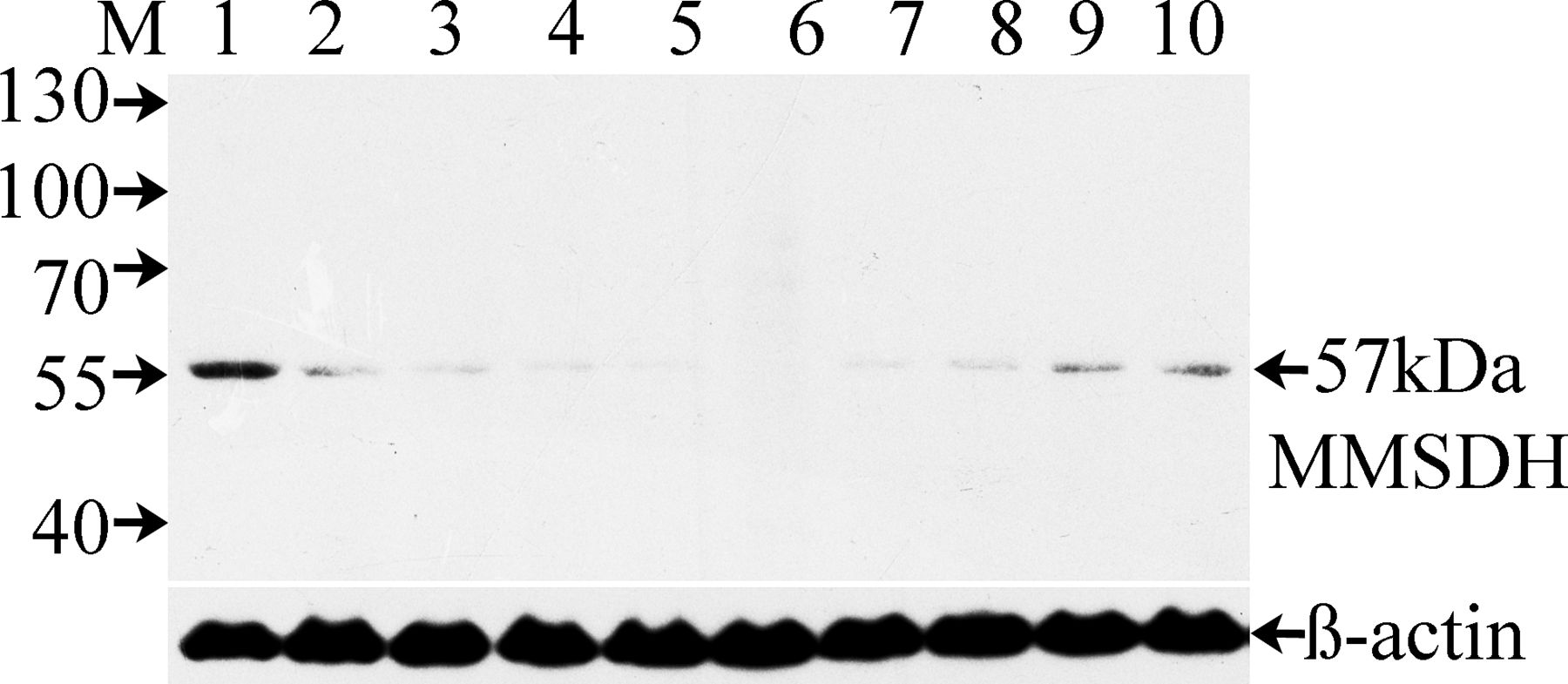
Western blot analysis illustrating effect of castration and supplementation with testosterone propionate. Lane 1 represents epididymal protein of day 10 sham-operated rats. Lanes 2–6 represent epididymal proteins from castrated rats 1, 3, 5, 7, and 10 days after surgery. Testosterone injections were given every day after 10 days postcastration, and the epididymal proteins were obtained at 1, 3, 5, and 7 days postinjection (lanes 7–10). The lower panel represents blot reactivity after stripping and reprobing with the anti–β-actin antibody (1:5000). Buffer-only and normal rabbit immunoglobulin G controls did not show any specific reactivity (data not shown). MMSDH indicates methylmalonate-semialdehyde dehydrogenase.
Developmental Expression
Toward understanding the developmental regulation of MMSDH expression in excurrent ducts, we followed the appearance of MMSDH in rat epididymis during postnatal development using Western blot analysis (Figure 5). The expression level of MMSDH was detected at relatively low levels from day 10 onward. There was a gradual increase of MMSDH expression from postnatal day 30 and the expression maximized at day 50 and remained at the same level on postnatal day 60. Negative control with no primary antibody, as well as normal IgG, did not show any reactivity. The lower panel of Figure 5 represents blot reprobed with β-actin antibody, and the signal indicates an equal load of protein in all lanes.
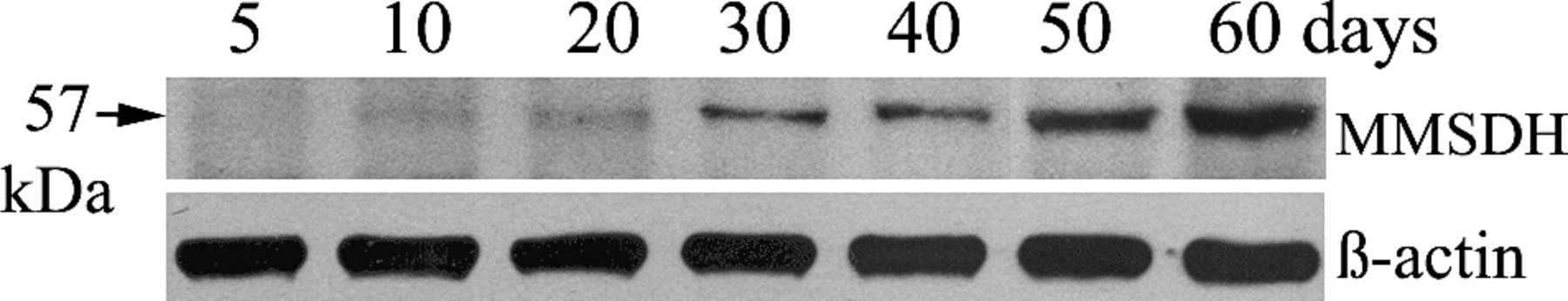
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis showing developmental expression of methylmalonate-semialdehyde dehydrogenase (MMSDH) protein. Rat epididymal proteins from day 5, 10, 20, 30, 40, 50, and 60 animals (lane 5, 10, 20, 30, 40, 50, 60) were subjected to SDS-PAGE and Western blot analysis using polyclonal antibody to MMSDH. Low levels were detected at day 10, and there was a gradual increase from day 30 that maximized at day 60. The lower panel shows blot reactivity after stripping and reprobing with the anti–β-actin antibody (1:5000). Buffer and normal immunoglobulin G controls did not show any reactivity (data not shown).
Mode of Anchoring on the Sperm Plasma Membrane
To gain insight into how MMSDH synthesized by the epididymal epithelium interacts with sperm in the lumen, we analyzed the mode of MMSDH anchoring on the sperm plasma membrane using sequential extraction with salt solution and detergent, as represented in Figure 6. When epididymal sperm were sequentially treated with an isotonic or a hypertonic salt solution, MMSDH at 57 kd was found to be present in the supernatants of PBS containing 150 mM, 1 M, 2 M, and 4 M NaCl (Figure 6A, lanes 1–4), indicating a loosely attached form of the protein. It was seen that the sperm treated with Triton X-100 showed an additional band at 62 kd (Figure 6A, lane 5), indicating the presence of a firmly bound form of MMSDH on the sperm plasma membrane. It was observed that all the pellet fractions after salt and Triton X-100 treatment when heated in Laemmli did not show any reactivity (data not shown). However, when these pellets of the treated fractions were extracted with 1% SDS, the form that was not released earlier after hypertonic solution treatment was detected at 62 kd (Figure 6B, lanes 1–5). Buffer alone and normal IgG, which served as negative controls, did not show any specific band (data not shown).
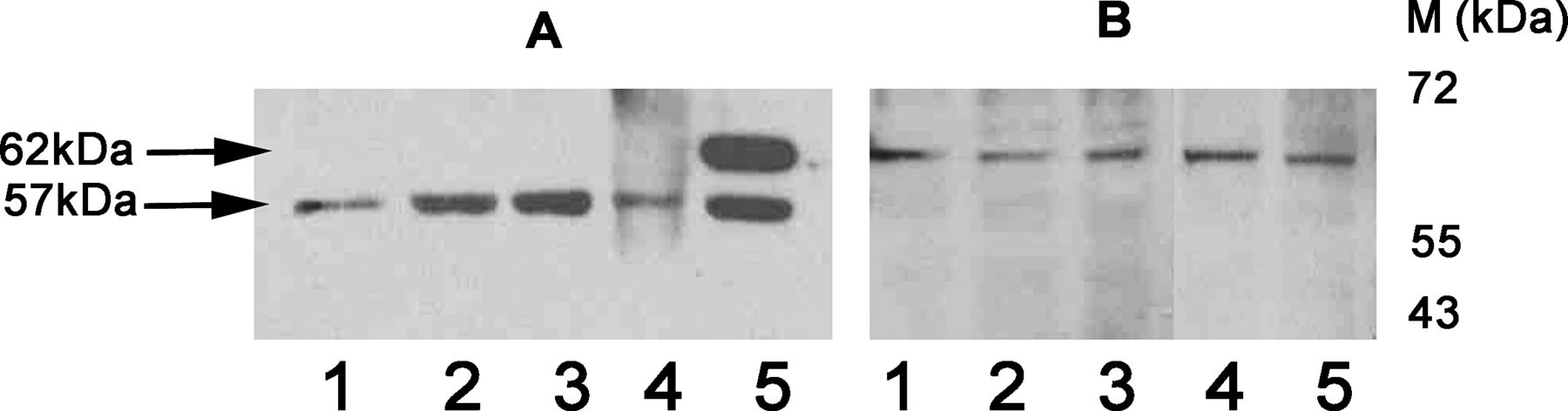
Sequential extraction of peripheral and integral proteins of the sperm using salt and detergent treatments. The blot was probed with the methylmalonate-semialdehyde dehydrogenase (MMSDH) antibody (1:25). (A)The presence of MMSDH at 57 kd in the supernatants obtained post—sequential extraction of sperm using phosphate-buffered saline containing NaCl at concentrations of 150 mM, 1 M, 2 M, and 4 M and with 0.1% Triton X-100 (lanes 1–5, respectively) indicate the peripheral form. An additional band at 62 kd was seen in the Triton X-100 supernatant (lane 5).(B) The pellet fractions (lanes 1–5) were subjected to 1% sodium dodecyl sulfate extraction and sonicated; the supernatant was heat denatured. The high-mass 62-kd integral form of the protein was detected in all lanes. Buffer control and normal immunoglobulin G did not show any specific reactive bands (data not shown).
Epididymosomes Show Presence of MMSDH and Mediate Its Transfer Onto the Sperm Membrane
Epididymosomes have been known to carry integral membrane proteins secreted by epididymal epithelial cells to spermatozoa. To determine whether the MMSDH protein was transferred via epididymosomes, Western blot analysis was done to check for its presence in epididymosomes from different epididymal regions. Results indicate the presence of MMSDH in epididymosomes obtained from the initial segment, caput, corpus, and cauda of the epididymis (data not shown). Analysis of the total epididymal fluid containing the vesicles showed the presence of the protein (Figure 7A, lane 2), and when the fluid was devoid of vesicles, it did not show any staining (Figure 7A, lane 4), indicating that the protein was present only in the epididymosomes (Figure 7A, lane 3) and that it might be secreted into the lumen by the principal cell of the epididymal epithelium. Presence of MMSDH in the epididymosomes raised the possibility that these membranous vesicles could act as carriers of protein to the sperm surface. To determine if this was truly the mode of protein transfer, testicular sperm was checked for MMSDH presence before and after co-incubation with epididymosomes from the cauda epididymis in the presence or absence of ZnCl2. Western blot analysis shows MMSDH acquisition by testicular sperm after co-incubation with epididymosomes in the presence of ZnCl2 (Figure 7B, lane 2) when compared with testicular sperm without epididymosomes (Figure 7B, lane 1). No reactivity of MMSDH was seen when the testicular sperm incubated in the absence of ZnCl2 with (Figure 7C, lane 2) or without (lane 1) epididymosomes from the cauda epididymis. An epididymal sperm lane (Figure 7A, lane 1) served as the positive control, showing reactivity to MMSDH. Blot probed with secondary-alone or normal IgG did not show any reactivity (data not shown). No cell death was noted during incubation in the presence or absence of zinc ions, indicating that the incubation conditions were not toxic to the sperm (data not shown).
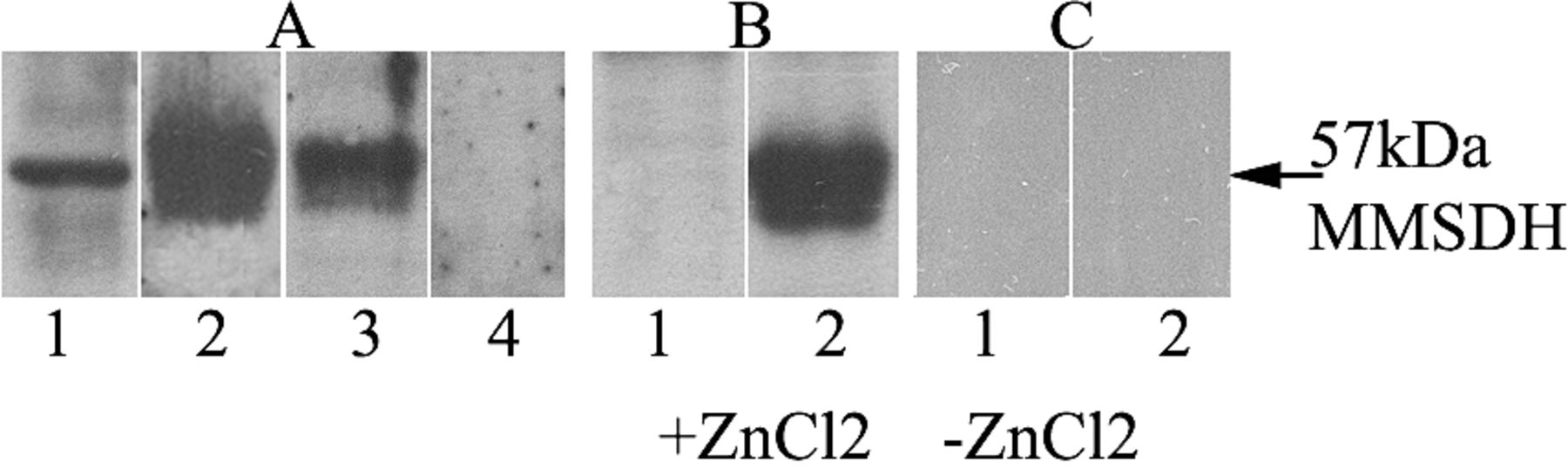
Western blot analysis indicating the presence of 57-kd methylmalonate-semialdehyde dehydrogenase (MMSDH) protein expression in epididymosomes and its acquisition by testicular sperm. (A) Blot shows the reactivity of MMSDH with epididymal sperm (lane 1), which also served as the positive control, and the epididymosomes obtained from the total epididymal fluid containing the epididymosomal vesicles (lane 2), the epididymosomal vesicles alone (lane 3), and the fluid devoid of the vesicles (lane 4). (B) Reactivity of MMSDH with the testicular sperm incubated in the presence of ZnCl2 without epididymosomes from cauda epididymis shows no specific band at 57 kd (lane 1), whereas reactivity of MMSDH with testicular sperm incubated in the presence of ZnCl2 with epididymosomes from the cauda epididymis is indicated (lane 2). (C) No reactivity of MMSDH was seen when testicular sperm was incubated in the absence of ZnCl2 without (lane 1) or with (lane 2) epididymosomes from the cauda epididymis. Blot probed with secondary-alone or normal immunoglobulin G did not show any reactivity (data not shown).
Discussion
Differential proteomics analysis indicated that MMSDH is present in both the head and flagellar domain of the sperm, and this was further confirmed by indirect immunofluorescence (IIF) localization (Suryawanshi et al, 2011). Sperm is supplied with a limited set of proteins and, to conserve energy, it is plausible that certain sets of proteins, such as MMSDH in this study, perform different functions by virtue of its localization on either the head or flagellum. A similar phenomenon has been noted in the case of sperm cytoskeleton protein TSA70 (Wakle and Khole, 2007). The IIF studies also showed presence of MMSDH on human sperm, indicating its conservation across species, which is a convincing proof of its importance in sperm function (Suryawanshi et al, 2011). In the present study, we have carried out further characterization of the MMSDH protein and evaluated its androgen dependence, developmental regulation, mode of anchoring, and acquisition on the sperm plasma membrane.
Western blotting and immunohistochemical results indicate that the protein is synthesized in the epididymis starting from the initial segment, with increasing levels throughout the length of the tubule. Not all cells of the epididymal epithelium are stained, indicating that the protein is synthesized and secreted by the principal cells. Almost 80% of the epididymal epithelium comprises principal cells, which are known to secrete several proteins and have been proposed to be involved in sperm maturation (Belleannee et al, 2009; Zhen et al, 2009). Principal cells appear to be by and large very active with respect to transfer and secretion of small organic molecules, absorption of fluid, and protein synthesis and secretion (Hermo et al, 2008). The MMSDH protein is synthesized in the principal cells and secreted in the lumen and probably exists in the soluble form of the protein, which is evident from the Western blot showing a band at 57 kd in the epididymal fluid lane until it is acquired by the sperm in the specific domain.
Temporal and spatial expression of the proteins in the epididymis is regulated by androgens. Androgen is vital for maintenance of spermatogenesis in the testis and for maturation of spermatozoa in the epididymis. Reduction in blood testosterone concentrations to undetectable levels and a corresponding decrease in MMSDH levels subsequent to castration indicate that testosterone could be involved in the modulation of MMSDH expression. Testosterone replacement for the animals 10 days after castration resulted in increased serum testosterone concentration and MMSDH expression levels in the epididymis, clearly indicating its androgen dependence. Similar observations have been reported for several epididymal proteins, such as transmembrane proteins, occludins and claudins (Cyr et al, 2007), Adam7 (Oh et al, 2009), Erabp (Fouchecourt et al, 2003), and Gstm2, Tpmt, and several others (Chauvin and Griswold, 2004).
The postnatal period from day 20 to 40 reflects the period before and after the rise in serum androgens. Postnatal days 49 and 56 are marked by the first appearance of spermatozoa in the caput and cauda epididymis, respectively. The significant increase in MMSDH protein expression observed during postnatal development of the rat epididymis have also been observed in other epididymal proteins, such as acidic epididymal glycoprotein (Charest et al, 1989), protein SP (Faye et al, 1980), and MTA1 (Ma et al, 2010). During maturation in the rat, the production of testicular testosterone, and perhaps its accessibility to the target organs, is amplified from days 20 to 40 (Gallon et al, 1989). On the other hand, the expression level of MMSDH was moderately low before postnatal day 20 and augmented considerably from postnatal day 30. This correlates very well with the attainment of hormonal maturation of the epididymis with increase in age, which has been demonstrated in several epididymal proteins. We have shown that MMSDH begins to appear in the epididymis as early as day 10 postnatal, and its expression continues to increase throughout the postnatal period to reach the highest levels shortly before puberty, which indicates that either its expression does not strictly depend on high androgen levels that are triggered during puberty or lower levels of circulating androgens in prepubertal male rats compared with adults are sufficient to trigger the progressive expression of MMSDH. The low levels of the protein detected in the prepubertal stage at day 10 does not rule out the possibility of its role in epididymal development, as seen in other epididymal proteins such as the HE1 and betagalactosidase—like gene Glb1l4, which is up-regulated distinctly at puberty until maturity (Uhlenbruck et al, 1993), with the later reported to be maximally expressed at puberty with a role probably in epididymal cell differentiation and regional maturity (Zhen et al, 2009).
MMSDH levels have been studied at both the mRNA andproteinlevelinseveralrattissues, such as kidney, liver, heart, muscle, and brain (Kedishvili et al, 1992). However, Western blot analysis of tissue distribution of MMSDH suggested that the protein might not correlate with mRNA distribution in all tissues, probably because of differences in translational control or protein degradation rates in different tissues. We have seen protein expression only in liver, kidney, thymus, and seminal vesicle.
Membrane proteins can be found loosely bound or firmly bound. When epididymal sperm were sequentially treated with salt and detergent solutions, MMSDH was found to be present in the supernatant, indicating the presence of the loosely bound form. The presence of the protein after 1% SDS extraction of the salt- and detergent-treated sperm pellet indicated the existence of a firmly bound form. Similar observations have been made for rat GP17 (Iusem et al, 1989) and mouse MEP-9, D/E, PBLP, and GPX5 (Rankin et al, 1992; Rejraji et al, 2002). For the epididymal protein CRISP1, it has been demonstrated that the loosely attached form extracted by salt extraction is similar to that released after capacitation, whereas the protein remaining on spermatozoa after capacitation and migrating to the equatorial segment corresponds to the tightly bound population or an integral form (Cohen et al, 2011). A similar mechanism could be proposed for MMSDH because it is known to have esterase activity (Popov et al, 1992). Presence of esterase in rabbit acrosomal extracts, testicular extracts, seminal plasma, epididymal fluid, and oviductal fluid was observed to remove the corona radiata from cumulus free rabbit ova during fertilization (Bradford et al, 1976). Esterase activity in mammalian spermatozoa has been reported previously in head, midpiece, and tail extracts of bovine sperm (Meizel et al, 1971) and in bovine (Bryan and Unnithan, 1972) and mouse acrosomes (Bryan and Unnithan, 1973) by cytochemical staining. However, we are the first to report MMSDH on rat and human acrosomes on specific sperm domains by IIF, indicating a specific function for them. The association of MMSDH esterase activity with the sperm head is of great interest, in that it is most likely involved in penetration of egg coats and membranes, as shown earlier (Allison and Hartree, 1970). However experiments to elucidate the role of the 2 forms of the protein in sperm capacitation and acrosome reaction need to be evaluated.
Epididymal proteins are secreted by either merocrine (ie, classical) or apocrine (ie, nonclassical) modes of secretion. Epididymal proteins with a signal peptide secreted in a merocrine fashion by the epithelial cells are anticipated to be soluble in the intraluminal compartment (Hermo et al, 1994). Sequence analysis using prediction tools indicate that the protein MMSDH lacks a signal peptide, but it has a transit peptide that probably directs the protein to the mitochondrial compartment. Proteins without a signal peptide have been proposed to undergo secretion by the epididymal epithelium through the nonclassical, apocrine mode, which is seen in several epididymal proteins, such as aldolase reductase, p26h, and macrophage migratory inhibition factor (Legare et al, 1999; Frenette et al, 2003; Sullivan et al, 2007). The proteins released by apocrine secretion are often GPI anchored (Nickel, 2003). Although it is predicted that MMSDH has no potential GPI anchoring site, it is also predicted that the best score for a GPI anchoring site is at the 520aa position of the protein sequence, which is not significant; however, this needs to be experimentally validated. The apocrine mechanism is characterized by projections, termed apical blebs, that encompass membranous vesicles of several proportions in the epididymis. The blebs separate from the cell surface, and secretory constituents are released when they undergo fragmentation. The released constituents then either become associated with the vesicles or dissolve in the extracellular region (Sullivan et al, 2007). In vitro experiments have shown that proteins such as P26h, P25b, and P34H, known to be involved in zona pellucida recognition, are synthesized in these regions, carried on to the sperm by epididymosomes, and later attached to the sperm surface via a GPI anchor (Legare et al, 1999; Sullivan, 1999).
To understand the mode of acquisition of MMSDH on the sperm, Western blot of epididymal vesicles from all the regions of the epididymis was done, and it was seen that all of them carry the MMSDH protein (data not shown). The protein secreted is probably transferred onto the sperm surface via the epididymal membranous vesicles present in the lumen, “prostasome-like particles,” or epididymosomes (Frenette and Sullivan, 2001). We found that MMSDH is transferred to testicular sperm in vitro. The conditions employed in this epididymosome-spermatozoa transfer are physiologically relevant because the pH of the epididymal fluid is 6.5 (Wales et al, 1966), and high concentrations of zinc are known to be present in epididymal tissues (Mawson and Fischer, 1951; Maldera et al, 2011). The presence of zinc in the fluid also has been reported to be essential for the association of proteins such as CRISP with the sperm (Maldera et al, 2011). The presence of MMSDH in epididymosomes and its transfer onto the sperm surface implicate its involvement in sperm maturation.
The outcome of our study suggests that MMSDH protein is present in the entire epididymis; its expression is regulated by androgen and the developmental status of the epididymis. The protein is associated with the epididymosomes, which mediate the transfer of the protein secreted in the lumen onto the sperm surface. Future studies deserve to be directed to further delineate the physiological relevance of this molecule in sperm capacitation and acrosome reaction essential for fertilization.




