Electroshocking-Induced Mortality of Four Fish Species during Posthatching Development
Present address: Department of Environmental Health Science, 201 Environmental Health Science Building, University of Georgia, Athens, Georgia 30602, USA.
Abstract
Immediate mortality of electroshocked fish was related to the stage of development after hatching. Larvae and juveniles of four species of fish were exposed to homogeneous voltage gradients (2–16 V/cm) of 60-Hz pulsed DC in water having an ambient conductivity of 100 μS/cm. Recently hatched fish and fish older than 100 d did not die after electroshocking. The developmental period most susceptible to electroshocking-induced mortality was near the time of transformation from larvae to juveniles for all four species. The highest predicted mortality occurred 22 d after hatching or at 19–21 mm total length (TL) for bluegill Lepomis macrochirus, 36–41 d or 29–32 mm TL for largemouth bass Micropterus salmoides, 42–43 d or 27–30 mm TL for channel catfish Ictalurus punctatus, and 38 d or 21 mm TL for Nile tilapia Oreochromis niloticus. During a 5-d observation period, there was no delayed mortality of channel catfish. A field validation experiment with 25-d-old largemouth bass (14–22 mm TL) indicated that mortality in the laboratory or around an electrofishing boat did not differ if voltage gradients were similar.
Introduction
In aquatic habitats where electrofishing is conducted, many species and life history stages of fish can be exposed to electric fields regardless of the objectives of the electrofishing operation. Fish can be injured by electric exposure (Hauck 1949; Reynolds 1996), but information about the relative susceptibility of different life stages of any fish species is sparse. Age-related differences in susceptibility to electrofishing-induced injury need to be determined because if sensitive developmental stages exist, such that the electric exposure could negatively affect fish populations, the electrofishing procedures should be modified.
Previous research on the negative effects of electrofishing on fish can be divided into lethal and sublethal categories. Sublethal effects include impacts on growth (Gatz et al. 1986; Dalbey et al. 1996), disease progression (VanderKooi et al. 2001), and reproduction (Muth and Ruppert 1996), but most commonly, studies have evaluated injuries related to vertebrae and associated tissues (Snyder 1992). In larger fish, the frequency and severity of spinal injuries increase with increasing fish length (Hollender and Carline 1994; Dalbey et al. 1996; Thompson et al. 1997), but injury has not been evaluated in larvae and newly transformed juveniles. The importance of spinal injuries on fish survival is unclear, and some studies indicate that grossly visible injury is not related to fish mortality after electrofishing (Spencer 1967a; Hudy 1985; Habera et al. 1996).
Several studies have concluded that only a negligible number of fish are killed during electrofishing and that fish mortality is not a concern (Hudy 1985; Barrett and Grossman 1988; Schneider 1992). Although laboratory experiments indicate that fish size is not directly related to mortality (Collins et al. 1954; Whaley et al. 1978), Habera et al. (1996) found that 7-d mortality was greater for rainbow trout Oncorhynchus mykiss less than 100 mm in total length (TL) than for larger fish. Electroshocking-induced mortality of salmonid (Godfrey 1957; Keefe et al. 2000), walleye Stizostedion vitreum (Newman and Stone 1992), and razorback sucker Xyrauchen texanus (Muth and Ruppert 1997) embryos has been documented, but little is known about the effects of electroshocking on other early life stages. In addition to experiments with embryos, Muth and Ruppert (1997) electroshocked yolk sac larvae (36 h posthatching) and found that growth, but not survival, was decreased. We know of no other field or laboratory study of the mortality of larvae and recently transformed juveniles.
The objective of our study was to evaluate electroshocking-induced mortality of life history stages from yolk sac larva to juvenile for largemouth bass Micropterus salmoides, bluegill Lepomis macrochirus, and channel catfish Ictalurus punctatus. Nile tilapia Oreochromis niloticus were also evaluated, but their developmental stages studied included only older larvae (transitioning to the juvenile stage) and young juveniles. All experimental fish were age 0, except that two of the test species (largemouth bass and channel catfish) were arbitrarily selected for comparing mortality of age-0 and age-1 fish. Additional objectives were to determine whether delayed mortality (5 d) occurred after channel catfish were electroshocked and to compare the mortalities of young largemouth bass in laboratory and field experiments.
Methods
Experimental animals
Adult largemouth bass, bluegills, and Nile tilapias were spawned in earthen ponds or fiberglass tanks at the Auburn University Fisheries Research Station, and channel catfish eggs were obtained from a commercial source (Blackbelt Aquaculture, Newbern, Alabama). Eggs were hatched in laboratory aquaria, and young largemouth bass and bluegills were fed zooplankton, brine shrimp (Artemia sp.), and fish feed (Ziegler Brothers, Gardners, Pennsylvania). Channel catfish and Nile tilapias were fed fish feed. Fish were kept in 40-L flow-through (0.25 L/min) aquaria receiving well water. The well water had the following characteristics: alkalinity, 25 mg/L as CaCO3; hardness, 23 mg/L as CaCO3; temperature, 18°C; pH, 7.1; dissolved oxygen, more than 6.0 mg/L; and ambient conductivity, 80 μS/cm.
Older largemouth bass (age, 13 months; mean TL, 222 mm [range, 176–300 mm]; N = 28) and channel catfish (age, 15 months; mean TL, 295 mm [range, 223–370 mm]; N = 31) were collected by seining ponds at the Auburn University Fisheries Research Station. Fish were kept for 3–5 d in 2,400-L fiberglass tanks receiving reservoir water (2–3 L/min) and were not fed after capture. The reservoir water had the following characteristics: alkalinity, 30 mg/L as CaCO3; hardness, 33 mg/L as CaCO3; temperature 25°C; pH, 7.1; oxygen, more than 6.0 mg/L; and ambient conductivity, 70 μS/cm.
Electric fields
Electric fields were generated by an electrofishing pulse box (TEG-10 Proto 1; Coffelt, Flagstaff, Arizona), modified to be powered by standard 110 V AC and to produce DC and square-pulsed-DC electric fields. Exposure chambers were plastic troughs (38.1 × 7.7 cm, 5.1 cm deep) for fish younger than 100 d; a 40-L glass aquarium was used for larger fish. Electricity was delivered to the water with aluminum plate electrodes, which conformed to the cross-sectional area of the troughs or glass aquarium. In the troughs, the electrodes were separated by 30 cm and could be moved from trough to trough. In the glass aquarium, plates were separated by 56 cm. Electric fields were measured with an oscilloscope each time a fish was exposed (THS 720A; Tectronix, Beaverton, Oregon). The oscilloscope was used to measure voltage amplitude, pulse frequency, and pulse width; all electrical measurements of experimental treatments were within 3% of the expected value. The voltage amplitude was used for determining the voltage gradient. All electroshocking experiments were conducted at 24–26°C and 98–102 μS/cm ambient conductivity; water conductivity was adjusted by adding artificial sea salts (Instant Ocean Synthetic Sea Salt, Aquarium Systems, Mentor, Ohio). Before beginning the experiments, we used the oscilloscope connected to a sampling electrode (Henry et al., in press) to test the homogeneity of the electric field in the plastic troughs and the glass aquarium; voltage gradients were uniform throughout the exposure chambers.
Laboratory experiments
Water temperature for small fish (4–60 mm TL) held in aquaria was adjusted to 25°C with aquarium heaters for 2–3 h before the aquaria were stocked with fish. Fish were taken from aquaria and stocked into troughs for 20 min before electroshocking. The plastic troughs had flow-through water exchange (well water) and plastic covers. During stocking, each fish was examined and any fish with apparent deformity, injury, or abnormal swimming was replaced. Recently hatched yolk sac larvae (2 d after hatching for channel catfish, 3 d after for bluegills and largemouth bass) were stocked at 10–50 fish/trough, and fish at ages 5–100 d after hatching were stocked at 1 fish/trough. Larger channel catfish (223–370 mm TL, 15 months old) and largemouth bass (176–300 mm TL, 13 months old) were stocked, one fish at a time, into the 40-L electroshocking aquarium 5 min before exposure.
Fish were exposed only once to a specific electric treatment of 60-Hz square-pulsed (3 ms) DC. The range of voltage gradients (peak) tested depended on the fish species and was selected based on the results of preliminary experiments: 2–8 V/cm for largemouth bass, 4–16 V/cm for bluegills and channel catfish, and 8–16 V/cm for Nile tilapias. Each treatment consisted of 3 replicate troughs for larvae no more than 3 d old; for fish older than 4 d, 10–40 fish (exposed individually) in each age-group were tested. Fish in troughs or the electroshocking aquarium were randomly selected to receive 20 s of electroshock or to serve as controls. Control fish had the aluminum plate electrodes in the water to simulate shocking, but the plates were not energized. For larvae 3 d or less old, lack of swimming was used to determine mortality; these fish were observed every 8 h for up to 24 h. Fish older than 4 d were considered dead if recovery of opercular movement did not occur within 1 h. After electroshocking, fish in the glass aquarium (176–300 mm TL largemouth bass and 223–370 mm TL channel catfish) were moved to a recovery aquarium (60 L, 1 fish/aquarium) containing flow-through reservoir water (described above) and were observed for 1 h.
After the 1-h observation period for fish older than 4 d, total length was measured, and small fish (4–60 mm TL) were preserved in 10% neutral buffered formalin or Bouin's fixative. Developmental stages of the preserved fish were determined by examining fish with a dissecting microscope. Criteria for stages were based on Kendall et al. (1984). The juvenile period for channel catfish began upon depletion of the yolk (Jones et al. 1978). For other species, fish were considered juveniles upon acquisition of the full complement of fin rays. Juveniles had a body form approximating that of the adult.
Delayed (5-d) mortality was evaluated only for channel catfish; 118 fish (age 51 d, 32–39 mm TL) were individually exposed to 4 V/cm 60-Hz pulsed DC (as described above). After 1 h, survivors were moved to 40-L glass aquaria with flow-through well water (as described above), and mortality was recorded daily for 5 d. At the same time, control fish were exposed to the same handling as electroshocked fish and were also moved to 40-L aquaria for observation for 5 d.
Field experiments
Field validation experiments were conducted in a pond (alkalinity, 25 mg/L as CaCO3; hardness, 28 mg/L as CaCO3; dissolved oxygen, 7.5 mg/L; ambient conductivity, 77 μS/cm; temperature, 28.7°C) located at the Auburn University Fisheries Research Station. An electrofishing boat was positioned in the pond with the anode dropper cables (1.0 m) suspended in water 1.0–1.3 m deep. Laboratory-reared largemouth bass (age, 25 d; 14–20 mm TL) were confined in cylindrical cages (0.3 m diameter × 0.7 m height) constructed of nylon mesh and plastic pipe. Cages were anchored at specific locations near the anode dropper cables. Fish were exposed for 20 s to 60-Hz pulsed DC generated by a Smith-Root (Vancouver, Washington) GPP electrofisher operated at 1000 V, 6.2 A, and 80% of full pulse width. During exposure, a sampling probe (Henry et al., in press) was used to measure the voltage gradient at one point near the center of the cage; the cage was located at different positions relative to the anode droppers to produce different voltage gradients within the cage. Three groups of 8–10 fish were exposed, and each group was exposed at a different location relative to the anode droppers (i.e., different voltage gradient). Control fish were handled the same way as electroshocked fish except for not being exposed to an electric field. Fish were left in the cage, and fish that had not recovered opercular movement after 1 h were considered dead.
One day after the field study, fish from the same source as used in the pond experiment were electroshocked (60-Hz pulsed DC) in plastic troughs (as described above) containing water from the pond (same water chemistry and temperature). Control fish were also transferred to the plastic troughs with pond water but were not exposed to an electric field.
Statistics
Statistical analyses were conducted with Statistical Analysis Software (SAS Institute, Cary, North Carolina). For yolk sac larvae no more than 3 d old (three replicate troughs, 10–50 fish/trough), the mortality of the electroshocked fish was compared with the mortality of the controls by analysis of variance. For older fish (exposed individually), we tested whether a quadratic function described changes in mortality rates and used logistic regression to model fish mortality as a function of fish age or (in a separate model) as a function of fish total length. Separate models were generated for each species and voltage level. The model was
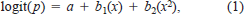
where logit(p) was the logistic probability of a fish dying; a was the intercept value; b1 and b2 were parameter estimates; and x was either fish age (d) or total length (mm). A model was considered significant if P < 0.05 based on the Wald chi-square.
The estimate of logit(p) was used to obtain the predicted probability of fish mortality (p):
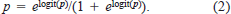
We used an index of rank correlation (c) to assess the predictive ability of the model (SAS Institute). The value of c indicates the percent effectiveness of the model for predicting a binary response. The most susceptible fish age (or TL) was calculated by setting the derivative of equation (1) equal to zero and solving for age (or TL) in each quadratic equation.
We used a G-test to compare the mortality of fish in the field validation experiment (Zar 1984). The results for fish in the pond were combined for two cages for which the voltage gradients differed by only 1 V/cm. A significance level of 0.05 was used to detect differences in fish mortality when fish were exposed to homogeneous electric fields in the laboratory or to heterogeneous electric fields in cages around an electrofishing boat.
Results
Electroshocking immobilized all species and ages of fish. All 2-d-old channel catfish began swimming within 2 min after electroshocking, but for 3-d-old bluegill and largemouth bass, observation for as long as 24 h after exposure was required to determine if each larva was alive. For fish older than 3 d that survived the electroshock, recovery of opercular movement and swimming ability generally occurred within 5 min, whereas all of the fish that died did not recover opercular movements at any time during the 1-h observation after the electroshock. There were no deaths of control fish older than 4 d.
The youngest largemouth bass that were electroshocked were 3-d-old yolk sac larvae; for this age, there was no difference (P = 0.65) in mortality between electroshocked and control fish. Mortality of 3-d-old largemouth bass yolk sac larvae was 13% for 2 V/cm, 17% for 4 V/cm, 9% for 8 V/cm, and 15% for controls. The yolk sac of largemouth bass was not visible by day 12, an age at which 40% of the fish died after exposure to 4 V/cm (Figure 1a). Electroshocking with 4 V/cm caused high mortalities throughout the late larval and early juvenile period until age 81 d, when no largemouth bass were killed by 4 V/cm. Electroshocking with 2 V/cm caused lower mortality and killed fish during a narrower range of developmental stages. No 13-month-old largemouth bass died after exposure to 8 V/cm (data not shown).
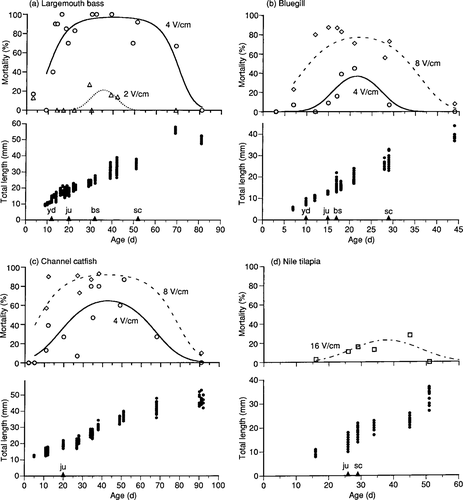
Relation of electroshocking-induced mortality to fish age (days after hatching), total length, and developmental stage for (a) largemouth bass, (b) bluegills, (c) channel catfish, and (d) Nile tilapias. Symbols indicate observed mortality, plotted lines predicted mortality. On the age axis, labeled points indicate stages and developmental events as follows: yd, yolk sac depleted; ju, juvenile (fin rays complete); bs, beginning of scale formation; sc, scale formation complete or nearly complete. No control fish died except for 3-d-old largemouth bass yolk sac larvae, which had 15% mortality.
Bluegill development and mortality (Figure 1b) were generally similar to those of largemouth bass, except that higher voltage gradients were required to obtain similar mortalities of bluegills. The youngest (3 d) and oldest (44 d) bluegills that were electroshocked with 8 V/cm had lower mortalities than did the intermediate ages. None of the 3-d-old yolk sac larvae died even after exposure to 16 V/cm. After exposure to 4 V/cm, mortalities were low for all ages except for newly transformed juveniles.
Channel catfish developed directly from yolk sac larvae to juveniles. The youngest channel catfish (2 d) that were electroshocked did not die (Figure 1c), even at 16 V/cm. Older yolk sac larvae and young juveniles exposed to 4 or 8 V/cm generally had high mortalities until day 91, when few fish died. There were no deaths of 15-month-old channel catfish exposed to 8 V/cm (data not shown).
The youngest Nile tilapias electroshocked were 16-d-old transitioning larvae; no yolk was present, all fin rays were present in all fins except the pelvic fins, and no scales were evident. There were no deaths at 8 V/cm; at 16 V/cm, the highest mortality was less than 30% (Figure 1d). The lowest mortalities for Nile tilapias were for the youngest and oldest age groups tested.
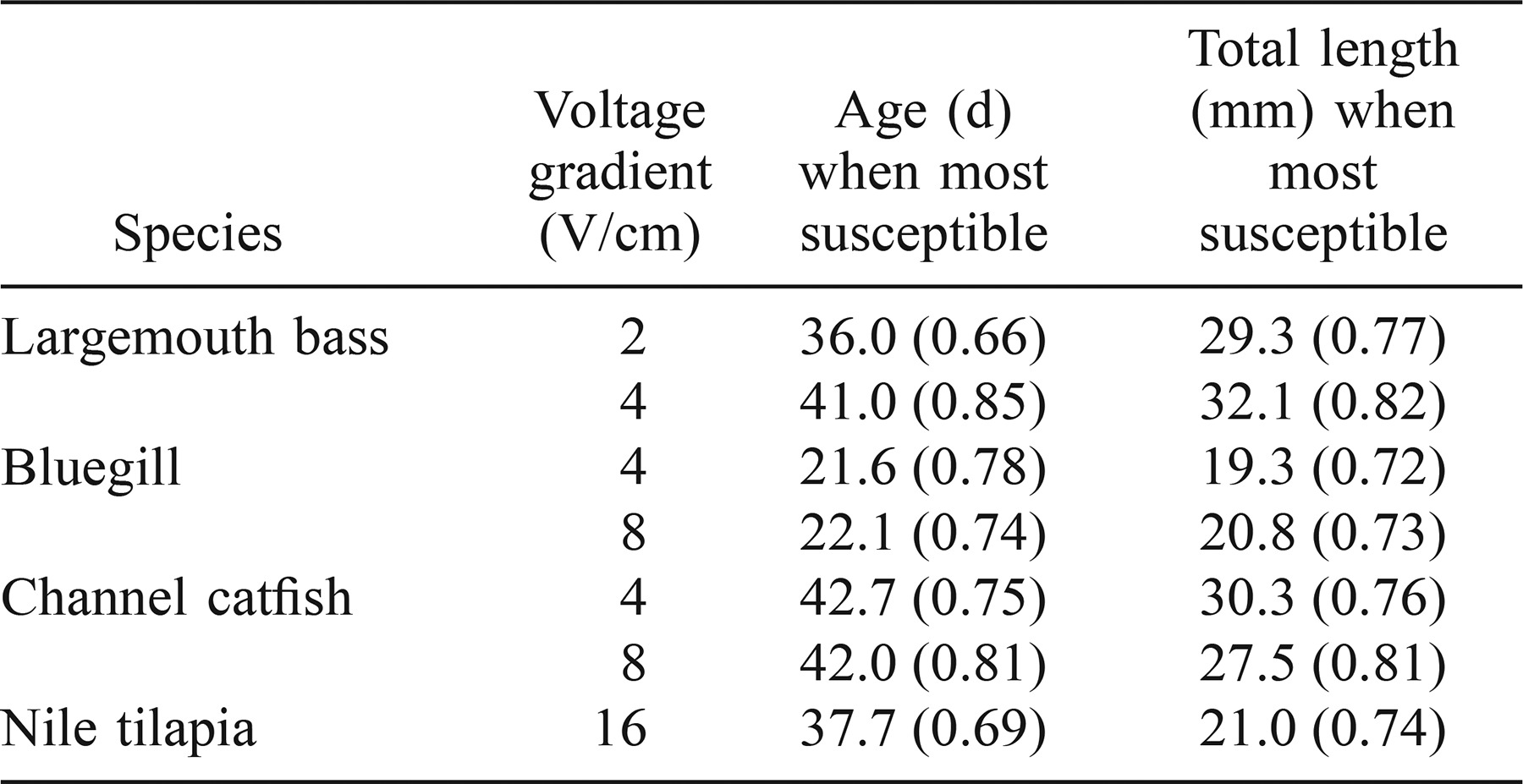
For each voltage gradient–species combination, a separate model of fish mortality was generated for both fish age and total length. All models were significant (Wald chi-square, P < 0.05) except for largemouth bass exposed at 2 V/cm (Wald chi-square = 3.98, P = 0.14) and Nile tilapias exposed at 16 V/cm (Wald chi-square = 4.18, P = 0.12). The index of rank correlation (c), which indicates the percent effectiveness of the model for predicting a binary response, was greater than 0.65 for all models (Table 1). The age with the highest predicted mortality was 42–43 d for channel catfish, 38 d for Nile tilapias, 36–41 d for largemouth bass, and 22 d for bluegills. The total length of fish with the highest predicted mortality was 28–30 mm for channel catfish, 21 mm for Nile tilapias, 29–32 mm for largemouth bass, and 19–21 mm for bluegills.
In the experiment to evaluate delayed mortality, 80 of the 118 electroshocked channel catfish did not recover opercular movement and were considered dead 1 h after electroshocking. None of the remaining 38 fish died during the 5-d observation period. No unshocked control fish died during the 5-d observation period.
Mortalities did not differ (G = 0.82, P > 0.05) among largemouth bass exposed to similar voltage gradients, whether generated by an electrofishing boat or in a homogeneous electric field in the laboratory (Figure 2). Higher voltage gradients caused higher mortalities in both situations, and all fish died after exposure to the highest voltage gradient. Exposure duration, ambient conductivity, temperature of the water, and fish size were similar for the field and laboratory exposures.
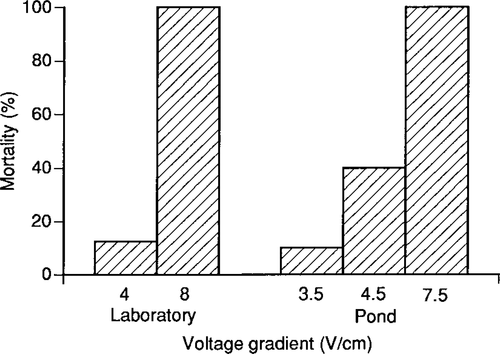
Field validation experiment with largemouth bass (age, 25 d; 14–22 mm TL; N = 9–12). For the laboratory experiment, fish in plastic troughs were exposed to a homogeneous electric field. For the pond experiment, an electrofishing boat was used to shock fish held in cages. Fish in both laboratory and pond experiments were exposed for 20 s to 60-Hz pulsed DC in water that had 77 μS/cm ambient conductivity
Discussion
For the four species electroshocked at different ages, electroshocking-induced mortality increased during late larval development or soon after metamorphosis to the juvenile stage. Models of fish mortality predicted that all of these species were most susceptible soon after becoming juveniles. For largemouth bass and bluegills, the most sensitive developmental stage was predicted to be before formation of scales was complete. Channel catfish were slightly older than largemouth bass and bluegills when they were predicted to be most susceptible; for Nile tilapia, the formation of scales was complete or nearly complete at the age at which predicted mortality was highest. The ontogenies of these species differ, and developmental differences among species could explain the relative differences we observed in which period had the greatest susceptibility.
Fish that died after electroshocking did not recover opercular movement at any time. No delayed mortality occurred for channel catfish observed for 5 d after exposure, even though 68% had died during the first hour after electroshocking. Previous studies reported low rates of delayed mortality (<10%) for other species (Hudy 1985; Barrett and Grossman 1988; Schneider 1992; McMichael 1993; Habera et al. 1996; Ruppert and Muth 1997; Cooke et al. 1998), although previous studies did not consider the developmental stages we found to be the most sensitive to the lethal effects of electroshocking.
Hypoxia is a potential cause of death when opercular movement is stopped after electroshocking. If hypoxia is the cause of immediate mortality after electroshocking, then the age-related differences in susceptibility we observed could be a reflection of developmentally related changes in tolerance for oxygen deprivation and the ability to obtain sufficient oxygen by way of cutaneous uptake. Oxygen uptake by fish is primarily cutaneous in embryos and young larvae, with branchial uptake becoming more important near the end of the larval period (Rombough 1988). For older fish, which depend on opercular movement to supply oxygen-rich water to the gills, a greater tolerance for insufficient oxygen uptake would improve chances for survival after cessation of opercular movement. Shepard (1955) found that the survival time of brook trout Salvelinus fontinalis was longer for larger fish after a sudden exposure to water with a lethally low concentration of dissolved oxygen. This suggests that after fish become dependent on branchial uptake of oxygen, cessation of opercular movements is more likely to be lethal to smaller fish.
Although each species experienced a period of high susceptibility to the lethal effects of electroshocking, the relative susceptibility appeared to differ among species. Some largemouth bass in the sensitive stages died after exposure to 2 V/cm, whereas 16 V/cm was required to kill Nile tilapias. Studies comparing the relative susceptibility of these species is a topic for further research.
Electroshock-induced mortality of fish has been evaluated in field studies (Pratt 1955; Bardygula-Nonn et al. 1995; Habera et al. 1996) and in laboratory studies with homogeneous electric fields (Collins et al. 1954; Whaley et al. 1978). The voltage gradients generated around electrofishing electrodes in field situations are heterogeneous; that is, intensity varies with the distance from an electrode (Kolz 1993). Determination of the voltage gradient and duration of exposure during electrofishing is difficult because of the large variation in electric fields (Henry et al., in press). Results of most field studies cannot be compared because voltage gradient and exposure duration, which are critical factors affecting electroshocking-induced mortality (Collins et al. 1954; Lamarque 1990), are unknown for most of these studies. In laboratory tanks, the effects of homogeneous electric fields can be determined, but the applicability of these results to electrofishing in field situations with heterogeneous fields has not been evaluated previously.
Mortalities of largemouth bass were similar in our experiment that compared heterogeneous and homogeneous electric fields. Voltage gradients were measured near the center of the cages during exposure of fish to the electric field produced by an electrofishing boat, but the minimum and maximum voltage gradients were not determined in these cages. However, confinement to a cage that was small relative to the overall size of the electric field kept the voltage gradient range small during our field experiment. We measured only the vertical and one horizontal component of the heterogeneous fields, which vary in three dimensions; by aligning the probe with the apparent maximum voltage gradient in the horizontal plane, however, the error resulting from our two-dimensional measurement was probably small compared with the variation expected within the cage (Kolz 1993). Although we found that laboratory experiments with homogeneous fields were useful for determining the lethal effects of electrofishing, additional validation of our results is needed.
In the present study, mortality of electroshocked fish varied in relation to the developmental stage of the fish; however, the relevance of this result to the practice of electrofishing will require additional evaluation. The importance of increased mortality of young fish on fish populations may be negligible for some species or even beneficial to reduce excess recruitment (e.g., centrarchid populations; Spencer 1967b), or may be viewed as highly detrimental for endangered species (Nielsen 1998).
Acknowledgments
The U.S. Fish and Wildlife Service provided funding for this project through a grant to the Fisheries Management Section of the American Fisheries Society. J. Boxrucker was instrumental to securing and administering the grant. Additional funding was provided by the Southeastern Cooperative Fish Disease Project. The authors thank A. Henry for advice on electrical theory and equipment. T. Arslan, R. Phelps, R. Carpenter, and T. Cole provided experimental fish. Laboratory assistance and care of experimental fish were provided by C. Wagner, J. Cochrane, and B. Beck. We thank C. Brunner, E. Irwin, and C. Johnston for reviewing the manuscript.




