Trophic Relationships among Lean and Siscowet Lake Trout in Lake Superior
Present address: Northwest Fisheries Science Center, National Marine Fisheries Service, Seattle, Washington 98112, USA.
Abstract
Lake trout Salvelinus namaycush occur in several different morphotypes in Lake Superior, including nearshore “leans” and deepwater “siscowets.” Siscowets, which are highly abundant and commercially undesirable, have been hypothesized to constrain populations of the more desirable lean lake trout through competition for prey. The stable isotope ratios (δ13C and δ15N) of leans and siscowets differed in several regions of U.S. waters, implying differences in both production base and trophic level. Spatial differences were partly a result of large-scale variation in food web structure, as stable isotope analysis of fishes in the genus Coregonus revealed different site-to-site patterns. In western Lake Superior, δ13C was similar among small leans and siscowets (implying a common production base) but diverged as the fish grew larger. The value of δ15N was greater for siscowets than for leans at all sizes, further implying that siscowets feed on a different prey assemblage than leans and are preying on another predatory fish, burbot Lota lota. A bioenergetics-based stable isotope model showed that siscowets in western Lake Superior may rely on nearshore prey for up to 25% of their production until they reach relatively large sizes. The large biomass of siscowets thus may exert strong predation pressure on nearshore communities in some regions. Evidence from central U.S. waters of Lake Superior, however, implies that siscowet predation on nearshore prey has not had a direct negative effect on lean lake trout stocks.
Introduction
Populations of lake trout Salvelinus namaycush in the Laurentian Great Lakes have changed dramatically since the early 1900s owing to fishing pressure, food web changes, invasion by the sea lamprey Petromyzon marinus, and hatchery stocking programs. Because of overfishing and sea lamprey predation, lake trout in the Great Lakes collapsed in the early to mid 1900s and were essentially extirpated from all of these lakes except Lake Superior (Christie 1974). The United States and Canada initiated intensive management efforts aimed at rehabilitation of this species, including sea lamprey control, fishery closures, and the stocking of fingerlings (Cornelius et al. 1995; Elrod et al. 1995; Eshenroder et al. 1995; Hansen et al. 1995; Holey et al. 1995). Despite these efforts, large-scale natural reproduction of lake trout has only been achieved in Lake Superior (Selgeby 1995). Lake Superior managers have continued efforts to reestablish self-sustaining populations approximating those of 1929–1943, and those efforts have been successful in some regions of U.S. waters (Wilberg 2000). However, in many regions of Lake Superior lake trout populations remain below target levels.
Among the possible limitations on lake trout rehabilitation is competition for a limited forage base, as bioenergetics models clearly demonstrate that lake trout growth rates are limited by prey availability (Ebener 1995; Eby et al. 1995; Negus 1995). Interspecific competition may occur between lake trout and exotic Pacific salmon Oncorhynchus spp. For example, rainbow smelt Osmerus mordax are important prey for both young lake trout (Mason et al. 1998) and Pacific salmon (Conner et al. 1993), and the relatively high growth and consumption rates of Pacific salmon result in high per capita forage demand (Negus 1995). However, Harvey and Kitchell (2000) found little evidence of dietary overlap between adult lake trout and Pacific salmon in western Lake Superior, and Kitchell et al. (2000) concluded that the salmon biomass in Lake Superior was insufficient to influence the biomass of forage species at large spatial scales.
Alternatively, intraspecific competition may occur between the major morphotypes of lake trout. Nearshore “lean” lake trout, which are valued by anglers and commercial fishers, inhabit regions between the shore and approximately the 80-m bathymetric contour of the lake, feeding primarily on lake herring Coregonus artedi, rainbow smelt, and slimy sculpin Cottus cognatus (Conner et al. 1993; Harvey and Kitchell 2000). Offshore “siscowet” lake trout, which are of low commercial value because of their high fat content (Eschmeyer and Phillips 1965; Kitchell et al. 2000), generally inhabit waters more than 80 m in depth and feed primarily on offshore, demersal coregonines (e.g., bloater Coregonus hoyi and kiyi C. kiyi) and deepwater sculpin Myoxocephalus thompsoni (Conner et al. 1993; Fisher and Swanson 1996; Harvey and Kitchell 2000). A third major lake trout variety, the “humper,” lives primarily on offshore shoals and is not discussed in this paper. There are accounts of siscowets making foraging bouts into shallower waters, which are the principal habitat of lean lake trout (e.g., Thurston 1962). Because siscowets are thought to outnumber leans by roughly 10-fold (Ebener 1995), such feeding bouts may negatively impact the available prey base even if they are uncommon or of short duration. This, in turn, could impose a constraint on lean lake trout population growth and hold stocks below their target levels in some regions of the lake.
Stable isotope analysis can be used to address the issue of dietary overlap between lean and siscowet lake trout and may help identify constraints on lean lake trout rehabilitation. Stable isotope ratios act as naturally occurring tracers that can reveal food web linkages (Peterson and Fry 1987). The stable isotope ratio of nitrogen (δ15N) is an estimate of the trophic position of a consumer because it increases strongly and predictably (∼3.4‰) with each trophic transfer up a food web (Minigawa and Wada 1984). Thus, top predators tend to be enriched in 15N compared with other members of a food web. Conversely, the stable isotope ratio of carbon (δ13C) experiences little or no increase (0–1‰) per trophic transfer, and thus the δ13C of a primary production base is retained in all consumers that it supports (DeNiro and Epstein 1978; Hecky and Hesslein 1995). The carbon isotope signature may thus reflect the location where an organism feeds: aquatic primary producers and the food webs they support can be relatively 13C depleted (as in deep waters, where respired CO2 is abundant) or 13C enriched (as in surface waters, where photosynthetic rates are high) (e.g., Hecky and Hesslein 1995). Because isotope ratios change (fractionate) predictably during trophic transfers, stable isotope data can be used in conjunction with stomach content analysis to infer long-term trophic relationships in a food web. In this way, the importance of a particular production base or prey species to a particular consumer can be examined and the potential for competition owing to diet overlap evaluated.
The goal of this study was to determine whether lean and siscowet lake trout experience diet overlap and thus whether siscowets may be constraining the rehabilitation of lean lake trout in Lake Superior. Although Harvey and Kitchell (2000) found little evidence of trophic overlap among lean and siscowet lake trout, their study focused only on the western waters of Lake Superior and did not account for temporal variation related to the dietary ontogeny of lake trout. In this study, we compared lean and siscowet lake trout in six areas and modeled temporal changes in the stable isotope ratios of siscowets exerting different levels of predation on nearshore forage species. Our analyses thus provide greater information on the spatial and temporal characteristics of diet among lake trout morphotypes.
Methods
Lake trout and coregonines were collected in bottom-set gill nets during the summer of 1997 in U.S. waters of Lake Superior (Figure 1). Lean lake trout and lake herring were taken at depths up to 80 m. Siscowet lake trout and deepwater coregonines (bloaters and kiyis, collectively called chubs) were taken at depths of 120 m or more. Where possible, at least five fish of each species were sacrificed and frozen following capture. Coregonines, a major item in the stomach contents of large lake trout in Lake Superior, were collected to improve the comparability of lake trout stable isotope ratios across regions. Cabana and Rasmussen (1996) showed that δ15N data from the base of a food web are necessary to standardize the δ15N of higher consumers before cross-site comparisons can be made. Although primary consumers (e.g., unionid mussels; Vander Zanden et al. 1997) would be better baseline organisms, we used coregonines because the primary consumers in deep benthic habitats were logistically impossible to collect.
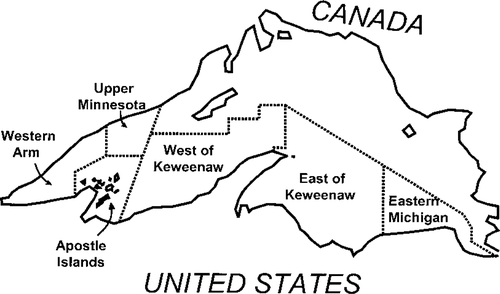
Map of Lake Superior showing general sampling regions
After fish were thawed and weighed to the nearest gram, dorsal muscle tissue was removed, dried to a constant mass at 60°C, and ground to a fine powder with a glass mortar and pestle. Nitrogen and carbon stable isotope ratios, percent nitrogen, and percent carbon for all tissue samples were determined using a Europa 20/20 continuous-flow mass spectrometer in series with a Carlo Erba CHN analyzer. The results are given as δ values, that is, as per mille deviations from standards (atmospheric nitrogen or Pee Dee Belemnite carbon) determined as follows:
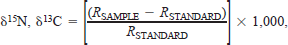
where R = 15N/14N or 13C/12C. Lipids in muscle tissues bias δ13C measurements because they fractionate carbon differently than proteins (DeNiro and Epstein 1978). Thus, lipids are often extracted from muscle tissues to determine unbiased δ13C values. We corrected our δ13C values using a model developed empirically from Lake Superior fishes, including coregonines, lean lake trout, and siscowet (C. J. Harvey, unpublished data), namely,
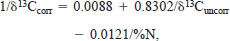
where δ13Ccorr is the δ13C value following lipid correction and δ13Cuncorr and %N are the δ13C and percent nitrogen values of the unextracted sample (n = 90, r2 = 0.86, P < 0.01). We developed this model by comparing the δ13C values of Lake Superior fish tissues before and after lipids were removed by petroleum ether extraction, as described in Harvey et al. (2002).
Stable isotope data for lean and siscowet lake trout were compared on a site-by-site basis to determine whether the two morphotypes experienced diet overlap. For this purpose, we used two-factor analysis of variance (ANOVA), with morphotype (lean versus siscowet) and region (see Figure 1) as factors. The stable isotope ratios of coregonines from nearshore (lake herring) and offshore (chubs) were also compared to qualitatively determine whether site-to-site differences in stable isotope ratios of the lake trout morphotypes were related to food web changes or spatial variation of isotopic baselines (e.g., Cabana and Rasmussen 1996; Vander Zanden et al. 1997), although we only had coregonine samples from four of the six regions.
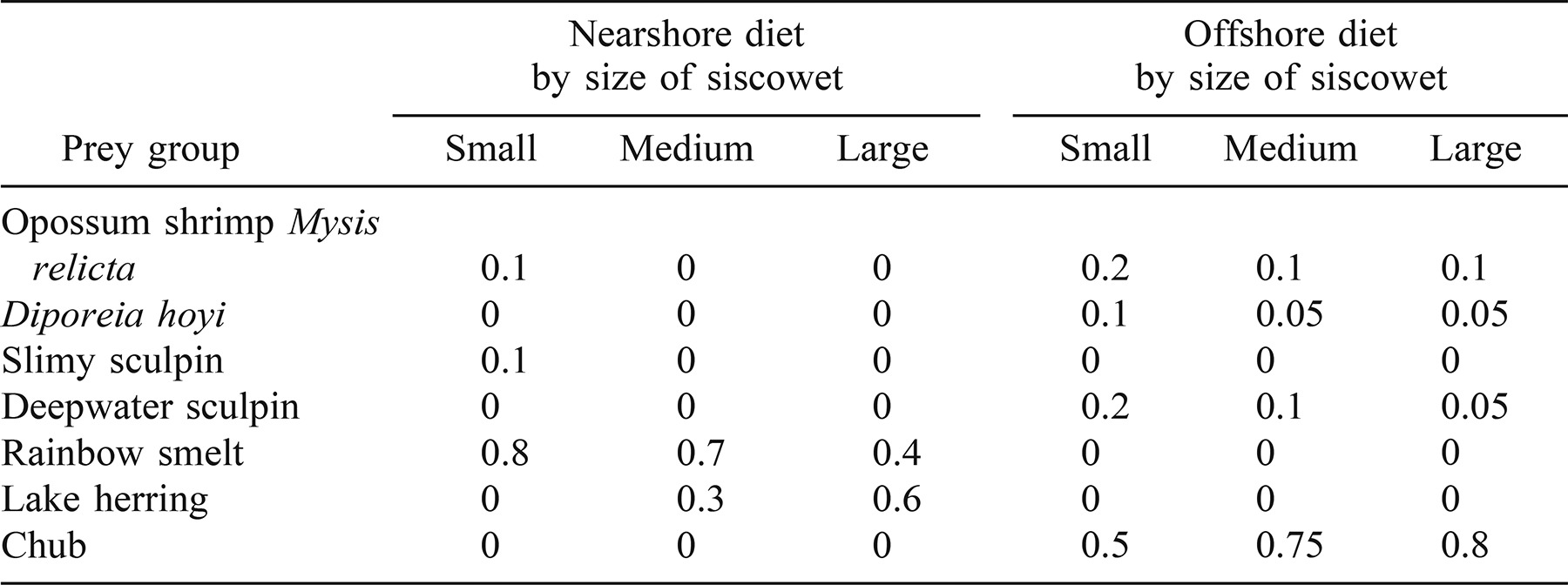
We used a dynamic stable isotope model to further examine diet overlap between lean and siscowet lake trout. Harvey et al. (2002) developed a bioenergetics model that incorporates stable isotope ratios and thus captures the temporal dynamics of those ratios as they are influenced by size-specific growth rates, temperature, and dietary ontogeny. The model includes uncertainty arising from the natural variation in isotope ratios, diet composition, and fractionation and thus generates distributions of estimated stable isotope ratios for the model predator. In essence, the model first predicts fish growth based on bioenergetics principles and then the likely distribution of a fish's stable isotope ratios based on its growth rate, diet, initial stable isotope ratios, and those of its diet (Harvey et al. 2002). The siscowet bioenergetics parameters are from the lake trout bioenergetics model of Stewart et al. (1983), the temperature data for siscowet habitat from Selgeby and Hoff (1996). In the simulations, siscowets grew from 454 g (age 7) to 3,765 g (age 21) based on size-at-age data from Ebener (1995). We created alternative models in which either 0% or 25% of the siscowet diet was nearshore prey typically consumed by lean lake trout (Table 1). The remainder of the diet was deepwater prey, an input that was based on stomach content data from juvenile and adult siscowet lake trout in western Lake Superior (Kitchell et al. 2000; Schram, unpublished data). Prey proportions were dependent on siscowet size (Table 1). To create the distributions of stable isotope ratios, we ran 1,000 iterations of the model for each of the alternative diets. In each iteration, the model randomly selected the initial stable isotope ratios for siscowets and their prey, along with a fractionation value. We used stable isotope ratios and standard deviations for western Lake Superior fishes and invertebrates (Harvey and Kitchell 2000) as the isotopic distributions for the diets (Table 2). The distributions for the initial siscowet stable isotope ratios were determined empirically (see Results). Trophic fractionation was assumed to be 3.4‰ (SD = 0.3) for 15N and 0‰ (SD = 1.1) for 13C. The model also randomized diet composition; the proportion of each prey item was drawn from a distribution in which the mean was the proportion in Table 1 and the standard deviation was arbitrarily set at 40% of the mean. The randomly drawn proportions were then rescaled so that they would sum to 1. Model outputs were compared with observed stable isotope ratios for siscowets from the Western Arm region (see Figure 1), a region from which we had relatively large sample sizes of lean and siscowet lake trout across a broad size range.

Results
Stable isotope ratios differed significantly between lean and siscowet lake trout as well as among lake trout in different regions of Lake Superior. According to the ANOVAs, δ15N was systematically greater for siscowet than for lean lake trout and δ13C was systematically lower (Table 3). Both isotope ratios differed among sites, but the lack of significant interaction terms indicates that isotopic differences between lean and siscowet lake trout were statistically consistent from region to region (Table 3). When examined graphically, however, isotopic differences between leans and siscowets appear to be relatively small within certain regions, particularly for δ15N in the East of Keweenaw region (Figure 2a) and δ13C in the West of Keweenaw region (Figure 2b). These and other regions had small sample sizes (Figure 2), which reduced the statistical power of the analysis. Thus, while the overall ANOVA results suggest lakewide differences between the two lake trout morphotypes, some fine-scale similarities may exist.
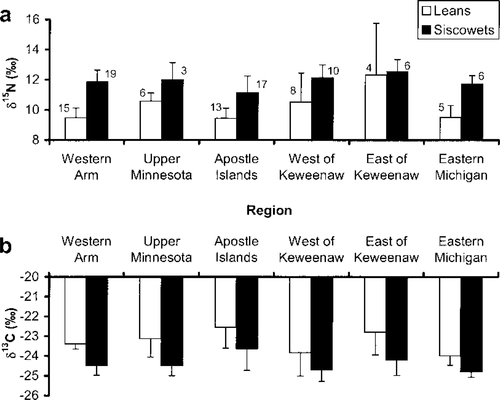
Mean stable isotope ratios of (a) nitrogen and (b) carbon for lean and siscowet lake trout captured in U.S. waters of Lake Superior. The ratios δ15N and δ13C are defined as [(RSAMPLE—RSTANDARD)/RSTANDARD] · 1,000, where R = 15N/14N or 13C/12C. Whiskers represent SDs; numbers are sample sizes
Stable isotope analysis may also have been confounded by field misidentification of lake trout. Lean and siscowet lake trout are sometimes difficult to distinguish based on external features alone, but they appear to have divergent amounts of carbon and nitrogen in their muscle tissues as they grow and siscowets become relatively fatty (Figure 3). As that figure indicates, some fish appear to have been misidentified. For example, the three highlighted fish, all of which came from the West of Keweenaw region, were identified as leans even though their carbon : nitrogen ratios are much more characteristic of siscowets. If these fish are reclassified as siscowets, the West of Keweenaw relationships depicted in Figure 2 change markedly, with the mean δ15N for siscowets far exceeding that for leans (12.2‰ versus 9.3‰) and the mean δ13C for siscowets depleted relative to that for leans (−24.8‰ versus −23.1‰).
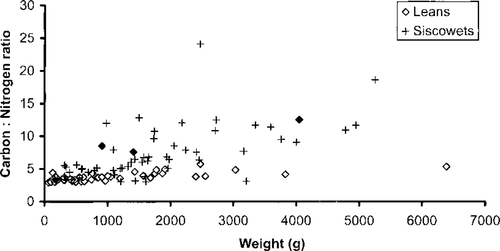
Size-specific carbon : nitrogen ratios in the muscle tissues of lean and siscowet lake trout from U.S. waters of Lake Superior. All values were recorded prior to lipid extraction. Solid diamonds indicate three lake trout from the West of Keweenaw region that were identified as leans but that have carbon : nitrogen ratios typical of siscowets (see text)
As implied in the ANOVA results, stable isotope ratios varied between regions of Lake Superior (Table 3); these results were further analyzed through post hoc contrasts (SAS Institute 1999) of geographically adjacent regions (see Figure 1). The only adjacent regions in which pooled lake trout stable isotopes were similar were the Western Arm and Upper Minnesota regions (P = 0.55 for δ13C, 0.13 for δ15N). All other adjacent regions showed differences for both isotopes (all P < 0.05), implying that relatively large-scale regional differences exist. Furthermore, the spatial patterns of coregonine stable isotopes were not the same as those observed in lean and siscowet lake trout (Figure 4). We only obtained coregonine samples from shallow (lake herring) and deep water (chubs) in four regions (Western Arm, Apostle Islands, East of Keweenaw, and Eastern Michigan), and sample sizes were poor in some regions (Figure 4), which compromises the interpretation of these data. However, the inconsistent spatial patterns suggest that the food web structure differs among regions.
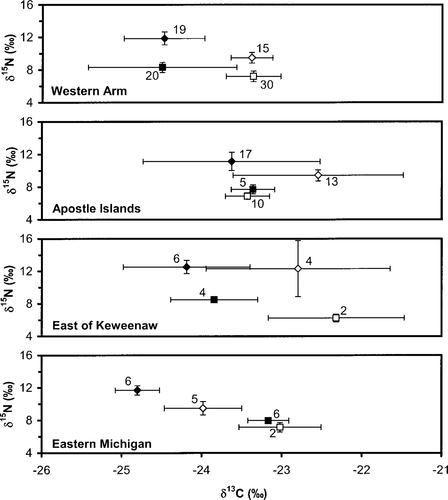
Mean stable isotope ratios for lean lake trout (open diamonds), siscowet lake trout (solid diamonds), lake herring (open squares), and chubs (solid squares) in four regions of U.S. waters of Lake Superior. Whiskers represent SDs; numbers are sample sizes
In the Western Arm region, the two lake trout morphotypes had different isotopic trends with respect to body size. There was little similarity in the δ15N of lean and siscowet lake trout of any size (Figure 5a). In contrast, small (<700-g) lean and siscowet lake trout had similar δ13C, implying a similar base of production, but diverged as they grew larger (Figure 5b). The δ13C for lean lake trout remained constant at about −23.4‰, whereas that for siscowets declined with increasing body mass before stabilizing at about −25‰. This decline was best described by the logarithmic function
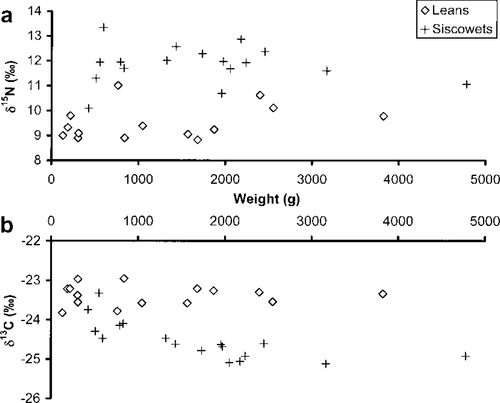
Observed stable isotope ratios of (a) nitrogen and (b) carbon for different sizes of lean and siscowet lake trout from the Western Arm region of Lake Superior

Bioenergetics-based stable isotope model outputs suggested that siscowets in the Western Arm region, particularly those of small to intermediate size, were obtaining a portion of their prey from nearshore waters. Although the simulations consistently underestimated δ15N relative to observed values (Figure 6a), the simulated values of δ13C followed trends similar to that of the observed data for siscowets (Figure 6b). The initial δ13C value for simulated siscowet lake trout came from the empirically derived δ13C-weight relationship (see equation above) for a 454-g fish. The initial δ15N value for simulated siscowet lake trout was 11.86‰, the mean of the empirical data in Figure 5. Standard deviations for the initial siscowet stable isotope signatures equaled those of the Western Arm region shown in Figure 2. Simulated fish that fed on 100% deepwater prey were 13C depleted compared with most observed siscowets, whereas those that fed on 25% nearshore prey and 75% deepwater prey had similar or greater δ13C values than most observed siscowets.
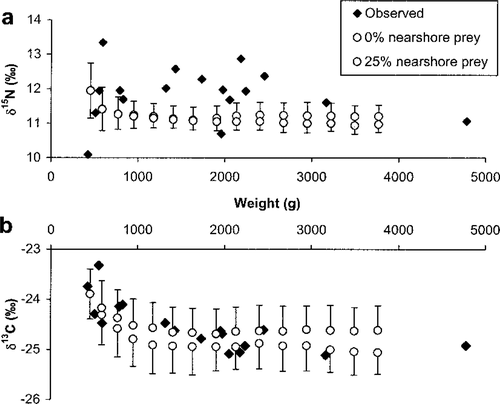
Observed and mean simulated stable isotope ratios of (a) nitrogen and (b) carbon for different sizes of siscowet lake trout in the Western Arm region of Lake Superior. Simulated fish consumed either 0% or 25% nearshore prey; all other consumption was of deepwater prey. Simulated fish were age 7 at the beginning of the simulation and age 21 at the end. Whiskers represent SDs
Discussion
The systematic differences between the stable isotope ratios of lean and siscowet lake trout imply a general lack of trophic overlap between the two morphotypes in Lake Superior. This implication supports previous findings. Harvey and Kitchell (2000) observed significant differences in the stable isotope ratios of lean and siscowet lake trout in western areas of Lake Superior and concluded that diet overlap was insignificant. However, that study assumed that both predators were in isotopic equilibrium with their diets, and it did not explicitly address potential predation on nearshore forage by siscowets. Furthermore, Harvey and Kitchell (2000) did not extract lipids from tissues and thus could not rigorously evaluate δ13C results. The present study addresses both shortcomings. We removed the potential bias of lipids in this study with the lipid-extraction correction equation. We also examined a broad range of lake trout sizes to follow ontogenetic changes in stable isotope ratios and showed that the two morphotypes may utilize a similar base of production during the early phases of their life histories in western Lake Superior. This does not necessarily indicate direct trophic overlap, however. Smaller lake trout showed similarity in only one stable isotope ratio (δ13C; Figure 5), which implies that they were utilizing the same production base but feeding at different trophic levels.
Based on model results, young to intermediate-age siscowets in the Western Arm appeared to acquire no more than 25% of their production from nearshore prey. This conclusion assumes that the diet proportions and δ13C values of prey adequately represent annual means. If the actual composition of the nearshore diet differs significantly from that in the model, the amount of nearshore prey in the siscowet diet is likely to be lower because the major nearshore prey consumed in model scenarios (rainbow smelt) is 13C depleted compared with other nearshore fishes (Harvey and Kitchell 2000). If relatively 13C-enriched fishes such as lake herring were more prevalent in the siscowet nearshore diet, simulated siscowets feeding on nearshore prey would experience a positive δ13C shift and the difference between model populations with 0% and 25% nearshore prey would increase. In any case, observed siscowets tended to be 13C enriched compared with those in the scenario with 0% nearshore prey, particularly among fish smaller than 2,000 g; this implies that siscowets rely partly on nearshore production for a considerable portion of their lives. This dietary history may exert a strong impact on nearshore ecosystems because the siscowet biomass in Lake Superior is very large (Ebener 1995; Kitchell et al. 2000).
The observed values of δ15N for siscowets in the Western Arm region were consistently greater than the model values. This implies that the δ15N of siscowet prey was variable, that our estimates of diet proportions were erroneous, or that there were other prey in the siscowet diet that were not included in the model. Kiriluk et al. (1995) observed seasonal variation in δ values of some Great Lakes biota, but such seasonal fluctuations should not be expressed strongly in top predators because of their relatively slow growth and turnover rates (Harvey et al. 2002). The second possibility, that the diet proportions in Table 1 were erroneous, is reasonable only if siscowets were consuming 100% chubs, which had the highest δ15N of any of the prey in Table 1 (8.3‰; Harvey and Kitchell 2000). Assuming a 15N fractionation of 3.4‰, a predator eating only chubs would thus have a δ15N of roughly 11.7‰. This is similar to the mean δ15N of siscowets in the Western Arm region (11.84‰). However, this scenario ignores the δ13C-based evidence that a portion of siscowet diet comes from nearshore prey, whose mean δ15N signatures range from 4.1‰ (the benthic amphipod Diporeia hoyi) to 7.7‰ (slimy sculpins) (Harvey and Kitchell 2000).
If there were other prey items that were not included in the model simulations, they would have to have δ13C values similar to those of other siscowet dietary items but occupy a higher trophic level and hence have elevated δ15N. A fish that meets these criteria is burbot Lota lota, which is occasionally found in siscowet stomachs (Conner et al. 1993; Ebener 1995; Fisher and Swanson 1996). Burbot are found at all depths of Lake Superior and thus may exhibit either a relatively enriched nearshore δ13C signature (e.g., Harvey and Kitchell 2000) or a relatively depleted deepwater δ13C signature. They are top predators, with δ15N values among the highest of Lake Superior fish species (Harvey and Kitchell 2000). Our results imply that their importance in siscowet diets may have been underestimated and that some siscowet production derives from predation on burbot. If burbot make up a portion of the nearshore diet of siscowets, that would further decrease the amount of direct trophic overlap between lean and siscowet lake trout, as burbot do not appear to be a major prey item for the former (Ebener 1995; Kitchell et al. 2000). In a food web model of Lake Superior, lean lake trout benefited from the removal of burbot (Kitchell et al. 2000), which may mean that siscowet predation limits burbot numbers and thereby facilitates lean lake trout recovery. Finally, it is possible that the δ15N of siscowets reflects some cannibalism, although burbot occur in lake trout stomach contents more often than do lake trout (Conner et al. 1993; Fisher and Swanson 1996).
While siscowets in the Western Arm region appear to have little diet overlap with lean lake trout, the degree of overlap in other U.S. waters of Lake Superior remains unclear. An interaction between the stable isotopes of the two morphotypes and the regions in which they were sampled may have been masked by small sample sizes or the misidentification of individual lake trout. We must therefore speculate about intraspecific trophic interactions in some regions. The East of Keweenaw region is an especially interesting case. Trophic overlap among lake trout morphotypes may occur there, as lean and siscowet lake trout samples were isotopically similar in that region. However, Wilberg (2000) found that lean populations in central Michigan waters, including the East of Keweenaw region, had returned to or exceeded the levels observed in 1929–1943, which are considered the target values for lean lake trout recovery (Hansen et al. 1995). Thus, if trophic overlap occurs between the two morphotypes in central Michigan waters, it has not prevented lean lake trout rehabilitation thus far. Evaluating lean and siscowet trophic overlap in this and other regions of Lake Superior would require substantially more samples covering a broader range of sizes so that the empirical and modeling approaches could be coupled, as was done in the Western Arm region.
The spatial patterns of lake trout stable isotope ratios were in part related to regional food web variation. Coregonine stable isotope ratios exhibited different spatial patterns than were seen in lean and siscowet lake trout, implying regional differences in food web structure. The large-scale spatial variation observed between adjacent regions can also be caused by differences in regional biogeochemistry that influence the isotopic signatures of nutrients available to primary producers. Spatial differences in both biogeochemistry and food web structure are likely to occur, given the size of Lake Superior and the patchiness of its fish populations (Hansen 1994). Point sources of nutrients may affect stable isotope ratios in some regions but not others (e.g., Hansson et al. 1997; Harvey and Kitchell 2000). Seasonal variation in nitrogen and carbon biogeochemistry may cause oscillations in isotope ratios, particularly among smaller organisms (Kiriluk et al. 1995; Cabana and Rasmussen 1996). However, the fishes considered here are large, long lived, and relatively slowly growing, so that their intrinsic growth characteristics should dampen the effects of seasonal isotopic oscillations in their diets (Harvey et al. 2002). Thus, the source of site-to-site changes in lake trout isotopes is probably a combination of spatially based biogeochemical variability and regional diet variation, with other prey (e.g., mysid shrimp, terrestrial insects, rainbow smelt, sculpins, and burbot) being more or less important depending on the site.
In conclusion, our results show that although lean and siscowet stable isotope ratios are generally distinct, some siscowets derive a portion of their growth from nearshore waters of Lake Superior. The latter finding is consistent with siscowet stomach contents, which often include nearshore prey such as terrestrial insects and rainbow smelt (M. P. Ebener, Chippewa–Ottowa Resource Authority, unpublished data). Given the considerable biomass of siscowets (Ebener 1995; Kitchell et al. 2000), the cumulative effect of their predation may represent a profound tax on nearshore food webs. This prey removal may affect lean lake trout rehabilitation in areas such as Wisconsin waters, where leans remain below target population levels. On the other hand, the isotopic similarity of leans and siscowets in central Michigan waters, where leans appear to have recovered to 1929–1943 population levels (Wilberg 2000), suggests that trophic overlap does not necessarily equate to intraspecific resource competition. Thus, while siscowets may influence the population dynamics of lean lake trout through indirect food web interactions, stable isotopes provide no clear evidence of trophic overlap among lake trout morphotypes, nor is there evidence that any such overlap restricts the rehabilitation of lean lake trout. Such conclusions should be revisited in the future: siscowet populations are expanding in some regions of Lake Superior (Horns, in press), and their role as nearshore predators and competitors with lean lake trout may change.
Acknowledgments
Sample collection was done by state, federal, and tribal agencies affiliated with the Lake Superior Technical Committee, and we are particularly indebted to Don Schreiner, Chuck Bronte, Mike Hoff, Mike Gallinat, Bill Mattes, Mike Donofrio, Jim Peck, Shawn Sitar, Ken Gephart, Mark Ebener, and the research crews at all affiliated agencies. Armand Krueger ran all stable isotope samples. Sean Cox, Blake Feist, Ashley Steele, Mary Moser, Daniel Hayes, Molly Negus, and two anonymous referees provided valuable comments on an earlier version of this manuscript. This research was funded by the University of Wisconsin Sea Grant Institute under grants from the U.S. Department of Commerce, National Oceanic and Atmospheric Administration (NA46RG0481 and NA86RG0047), and the state of Wisconsin.




