Critical Thermal Maxima of Wild and Domestic Strains of Trout
Present address: Post Office Box 386, New Fairfield, Connecticut 06812, USA.
Abstract
The slow growth and high mortality of hatchery-reared brown trout Salmo trutta and rainbow trout Oncorhynchus mykiss during summer in a stream that supported wild brown trout prompted us to compare the critical thermal maxima (CTMax) of wild and domestic strains of brown trout, brook trout Salvelinus fontinalis, and rainbow trout. First, we tested the effects of electrofishing exposures on the CTMax of hatchery-reared brown trout. We compared the CTMax of three domestic strains of brown trout to determine possible genetic influences on upper temperature tolerance and then collected wild trout with electrofishing gear and compared their CTMax to that of the hatchery strains. There was no significant effect of electrical shocks on the CTMax of hatchery-reared brown trout, and CTMax differed significantly among these three domestic strains. Wild strains of all three species had significantly higher CTMax than did domestic strains; differences ranged from 0.5°C to 1.6°C. We tentatively conclude that differences in CTMax between wild and domestic strains are genetically based.
Introduction
The impetus for this project was provided by two observations made by the first author during studies of a central Pennsylvania stream (Spring Creek) that supports a dense population of wild brown trout Salmo trutta. In one study, water from Spring Creek was continuously pumped into aquaria with a domestic strain of brown trout. During summer when stream temperatures reached 20°C, there were outbreaks of several diseases among the domestic trout and most died within a few days. Subsequent attempts to hold domestic brown trout at ambient stream temperatures during summer resulted in high mortality. The relative effects of high temperature and disease could not be determined. In another study, two strains of domestic rainbow trout Oncorhynchus mykiss were stocked in a section of Spring Creek that had a relatively low biomass of wild brown trout. During the summers of 1991 and 1992, growth of rainbow trout was substantially less than that of wild brown trout. These results prompted us to question the tolerance of domestic strains of trout to high summer temperatures.
A fish population's temperature tolerance is a function of its genetics and its thermal history or acclimation. The relative importance of these two factors varies among species. Vincent (1960) provided convincing evidence that the upper temperature tolerance of wild brook trout Salvelinus fontinalis was greater than that of a domestic strain owing to genetic factors when he reared eggs from both strains under identical conditions and then measured temperature tolerance. Wahl's (1974) work with domestic strains of brook trout from Pennsylvania hatcheries showed significant differences in upper temperature tolerances, which supports the notion of genetic influence, but he did not compare domestic strains with wild ones. Kaya (1978) compared upper temperature tolerances of wild rainbow trout from the Firehole River, a geothermally heated stream in Wyoming, with two hatchery strains and found no difference, suggesting that wild rainbow trout had not developed an enhanced ability to withstand high temperatures. Konecki et al. (1995) found no differences in upper temperature tolerance of coho salmon Oncorhynchus kisutch from three streams with substantially different maximum summer temperatures. Similarly, Lohr et al. (1996) showed that the upper temperature tolerances of Arctic grayling Thymallus arcticus from the Big Hole River in Montana, in which maximum summer temperatures approach lethal levels, were similar to those for an Alaskan population of Arctic grayling; they concluded that exposures to high summer temperatures in the Big Hole River had not led to genetic divergence of these populations. Thus, among salmonids there is some evidence of a genetic influence on upper temperature tolerance, but there is no evidence that thermal history is important.
Among nonsalmonid species, the relative importance of thermal history and genetics on upper temperature tolerance is mixed. Feminella and Matthews (1984) found that orangethroat darters Etheostoma spectabile from streams with widely fluctuating temperatures had higher temperature tolerances than fishes from springs with relatively constant temperatures. In contrast, Matthews (1986) compared populations of red shiners Cyprinella lutrensis collected along a 1,100-km north–south gradient and found no difference in upper temperature tolerances, suggesting that thermal history had little effect. Other studies indicate a genetic basis for differences in temperature tolerance. Amargosa pupfish Cyprinodon nevadensis from rivers with widely varying temperatures had higher temperature tolerances than populations from springs with relatively constant temperatures, and rearing experiments confirmed genetic divergence among these populations (Hirshfield et al. 1980). Meffe et al. (1995) showed that eastern mosquitofish Gambusia holbrooki from a thermally heated pond had higher temperature tolerances than fish from ambient temperature ponds. Eastern mosquitofish from the heated pond that survived exposures to high temperatures had a greater degree of heterozygosity than fish that did not survive, which suggests selection for fish with higher temperature tolerance within this population. Hence, among a wide diversity of fishes displaying differences in thermal tolerance among populations, there is evidence for genetic and environmental influences.
Temperature tolerances of fish and other ectotherms are estimated using static methods, which measure time to death at constant test temperatures, or dynamic methods, which increase test temperatures until a well-defined endpoint is reached (Lutterschmidt and Hutchison 1997). For this study, we chose a dynamic method and measured the critical thermal maximum (CTMax), because this index has become the most widely used to assess relative thermal tolerance (Beitinger et al. 2000). Our objectives were to compare CTMax of four domestic strains of brown trout to determine if upper temperature tolerance had a genetic basis and to compare CTMax of domestic and wild strains of brown trout, brook trout, and rainbow trout.
Methods
All experiments were conducted from May 1994 through May 1995 at the Benner Spring Fish Research Station, which is operated by the Pennsylvania Fish and Boat Commission (PFBC). Domestic trout were obtained from several PFBC hatcheries (Table 1), which have alkaline (about 150 mg/L as CaCO3) water supplies delivered by combinations of springs and wells in limestone aquifers. There had been little or no exchange of broodstock among these hatcheries in the previous 15–20 years.
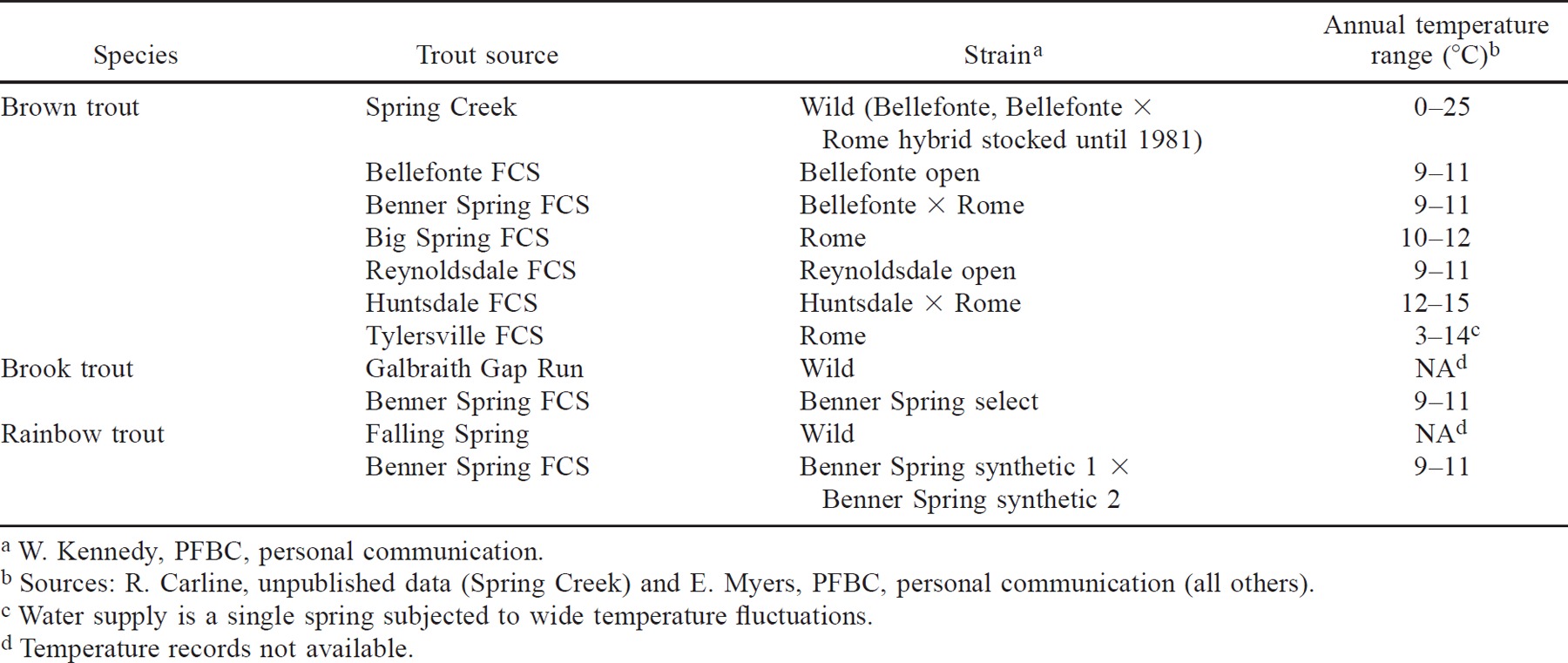
Because we planned to use electrofishing to collect wild trout, we conducted a preliminary experiment to determine if electrofishing affected the CTMax of four strains of domestic trout. Groups of 100 domestic brown trout were obtained from the Benner Spring, Big Spring, Huntsdale, and Reynoldsdale Fish Culture stations (FCSs) and transported to the research facility in late May 1994. We used age-0 domestic trout (total length range: 65–112 mm) in this and all subsequent tests.
Each strain received a different finclip and was separately held under a 12:12 photoperiod in 53.9-L acclimation tanks for 2 weeks before testing. Mean acclimation temperature for these groups was 10.9°C. Tanks received an inflow of 7 L/min of spring water (weekly temperature variation of < 0.2°C). All fishes were fed a commercial trout diet at 1% of their body weight per day.
We electroshocked one strain at a time by placing 50 fish in a 400-L fiberglass tank filled with 10.9°C water. The fish were electroshocked for 1 s with 100 V of pulsed DC from a backpack electrofisher (Coffelt model BP-1C). The adipose fin of each shocked fish was clipped before returning it to the acclimation tank. The remaining 50 fish of each strain were placed in the fiberglass exposure tank and then returned to the acclimation tank without receiving an electroshock or an adipose clip.
From 4–14 d after electroshocking, the CTMax of shocked and unshocked groups of each strain were measured. We conducted 10 CTMax trials; one to three trials were run on a given day. A trial consisted of four tests (one per strain). Three fish from one strain (one shocked and two unshocked or two shocked and one unshocked) were used in a test. In all, 30 fish/strain were tested, 15 shocked and 15 unshocked.
All CTMax tests were conducted in an aerated 38-L glass tank. Three fish were placed in the test tank and held at acclimation temperature for 30 min to reduce stress from handling. During this time, well water flowed through the tank at 0.5 L/min. Throughout the experiment, water temperature in the test tank was recorded every 5 s by a Ryan TempMentor (model RTM) with a neoprene coated bead sensor. Dissolved oxygen concentration was monitored every 5 min with a Yellow Springs Instruments model 58 m. During the test, an SWHX copper heat exchanger (NESLAB Instruments Inc.) increased the test tank temperature by 0.3°C/min. The test endpoint for a fish occurred when it lost the ability to remain upright for at least 5 s, at which time the temperature was recorded and the fish was removed from the tank, weighed, and measured for total length.
Wild trout were collected from three streams using a pulsed-DC backpack electrofisher that was adjusted to produce an output of 100–125 V. Fish were transported to the research facility in a tank of aerated stream water.
The Benner Spring, Bellefonte, and Tylersville Fish Culture Stations each provided about 100 domestic brown trout in late August 1994, and 88 wild brown trout were collected in September 1994 from a reach in Spring Creek that is 13 km upstream of the Benner Spring Fish Culture Station. Brown trout have not been stocked in Spring Creek since 1981 (T. Greene, PFBC, personal communication); hence, we are relatively certain that these fish were wild. Domestic (length range 90–183 mm) and wild brown trout (age 0, length range 74–126 mm) were held in acclimation tanks at 12.0°C and CTMax tests were conducted from October 21 through November 2, 1994.
We collected about 40 wild brook trout from Galbraith Gap Run, a tributary to Spring Creek, in early December 1994. This stream has never been stocked by the state (T. Greene, PFBC, personal communication). Domestic brook trout were obtained from the Benner Spring Fish Culture Station. Brook trout were acclimated at 10.6°C and were tested December 16–19, 1994.
We collected about 40 wild rainbow trout (age 0; length range, 95–140 mm) from Falling Spring, Franklin County, Pennsylvania, in January 1995. This section of Falling Spring has not been stocked by the state since 1977 (T. Greene, PFBC, personal communication). Domestic rainbow trout (length range 138–222 mm) were obtained from the Benner Spring Fish Culture Station. All rainbow trout were held at 9.8°C and were tested February 9–17, 1995. In CTMax tests of wild and domestic strains of each species, we conducted 10 trials or one trial per strain and used three fish (same strain) per trial.
We first determined if data followed a normal distribution by using the Anderson–Darling test and used analysis of variance (ANOVA) to test equality of mean CTMax for shocked versus unshocked fish and wild versus domestic strains. When analyzing data, we used the CTMax of individual fish in the electroshocking trials because we used fish from both treatments in each test. In comparing wild and domestic strains, we used three fish from the same strain per test, and the mean CTMax of these three fish was used in the analyses. Pearson correlation coefficients for length and CTMax and condition factor (K) and CTMax were calculated with data for individual fish for each strain. Statistical significance was set at α = 0.05; all analyses were completed with Minitab software (Minitab 2000).
Results
Among the 16 groups of trout, distributions of CTMax deviated from normality in only two of these groups: unshocked brown trout from Benner Spring FCS (P = 0.02) and brown trout from Tylersville FCS (P = 0.003). Because the CTMax of nearly all groups approximated normal distributions, we did not transform any data.
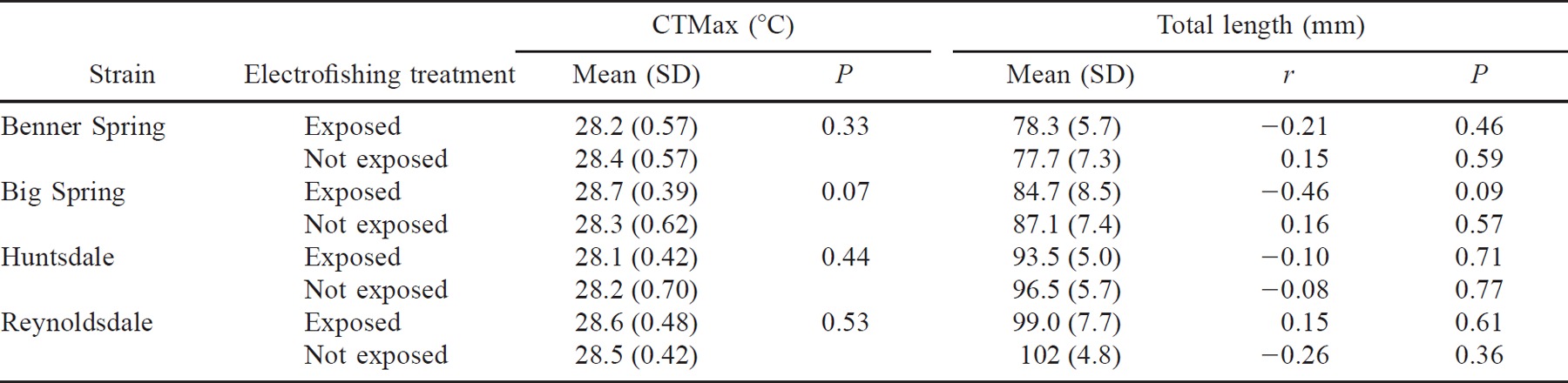
Electrical shocks from DC electrofishing gear did not affect the mean CTMax of any of four strains of domestic brown trout (Table 2). Within each strain, the mean CTMax values of shocked and unshocked groups did not differ (ANOVA, P > 0.05). In addition, CTMax and length (range, 65–112 mm) were not correlated within the shocked or unshocked groups of each strain.
Among brown trout strains acclimated at 12.0°C, the mean CTMax of wild trout (29.0°C) was 0.9–1.7°C greater (ANOVA, P < 0.001) than the mean CTMax of three domestic strains (Table 3). Among the domestic strains, the Benner Spring (28.1°C) and Tylersville (28.0°C) strains had higher mean CTMax values than the Bellefonte strain (27.3°C).

The mean CTMax for wild brook trout was 0.5°C higher than that of domestic brook trout (ANOVA, P = 0.001; Table 3). Similarly, the mean CTMax for wild rainbow trout was 0.5°C higher than that of domestic rainbow trout (ANOVA, P = 0.014; Table 3). Thus, wild trout of all three species had higher CTMax values than domestic strains.
There was no indication that length or condition influenced CTMax. When we correlated CTMax and total length for each of the eight test groups, two correlations were significant (P < 0.01; Table 3). The CTMax of Bellefonte strain brown trout was negatively correlated with length, whereas the CTMax of wild brook trout was positively correlated. In all tests, the mean condition factor (K) of stream-dwelling fishes was lower (ANOVA, P < 0.05) than that of hatchery fishes (Table 3), but none of the correlations between CTMax and condition factor were significant (P < 0.05). Hence, we concluded that results of CTMax tests were not confounded by fish size.
Discussion
We believed that our use of electrofishing to collect wild trout was acceptable because we found no effects of electrical shocks on the CTMax of brown trout. The only other published study of this type was conducted by Strange et al. (1993), who subjected rainbow trout to two AC exposures 5 min apart and then measured CTMax 25 min later. The exposed group had a significantly higher CTMax (30.0°C versus 29.4°C) than the controls. This result was unanticipated because one would expect a stressor such as electrical shock to reduce, rather than increase, upper temperature tolerance. An important difference between our study and that of Strange et al. (1993) was that we allowed trout 4–14 d to recover from stress produced by electrical shocks, whereas Strange et al. (1993) tested trout shortly after shocking them.
It is generally accepted that, for a given fish species, CTMax increases with increases in acclimation temperature (Lutterschmidt and Hutchison 1997). Becker and Genoway (1979) working with coho salmon and Currie et al. (1998) working with rainbow trout showed that CTMax was postively related to acclimation temperature. We would have expected the observed CTMax in this study to be higher for both strains of all species had the acclimation temperatures been higher; but the literature does not suggest that the difference in CTMax between two groups of fish would change with acclimation temperature.
Heating rate during a CTMax trial is also positively related to CTMax (Becker and Genoway 1979; Currie et al. 1998). We used a heating rate of 0.3°C/min, which was recommended by Becker and Genoway (1979) and Currie et al. (1998), though others have recommended rates up to 1.0°C/min to minimize possible acclimation during the test (Lutterschmidt and Hutchison 1997). Here again, we would anticipate a higher CTMax at faster heating rates, but there is no reason to suspect that a faster heating rate would alter the differences between wild and domestic strains.
Our results consistently showed that the CTMax for wild strains was higher than that for domestic strains, which leads one ask whether these differences were related to genetic divergence between strains or to differences in prior exposures (i.e., wild trout had been exposed to substantially higher temperatures than hatchery-reared trout). Studies with orangethroat darters (Feminella and Matthews 1984), bluegills Lepomis macrochirus (Holland et al. 1974), and several subspecies of Amargosa pupfish (Brown and Feldmeth 1971) have shown that populations from environments with widely fluctuating and high temperatures had higher CTMax values than populations from environments with relatively constant and lower maximum temperatures. However, similar studies with salmonids have failed to show an effect of environmental acclimation on CTMax (Kaya 1978; Konecki et al. 1995; Lohr et al. 1996). Beitinger and Bennett (2000) compared incipient lower and upper lethal temperatures for 21 temperate fishes and found that the upper temperature tolerance of brook trout was least affected by acclimation. These authors were not able to compare rainbow trout and brown trout, though several species of Oncorhynchus showed less influence of acclimation on upper temperature tolerance than did nonsalmonids. If acclimation temperature does not strongly affect the upper temperature tolerance of salmonids, genetic factors may have the most influence.
Wahl's (1974) study of 31 strains of domestic brook trout and our results from several domestic strains of brown trout suggest a genetic basis for differences in upper temperature tolerance. Presumably, these genetic differences developed as hatchery strains were selected for traits such as disease resistance, growth, and feed conversion, but not for high temperature tolerance. In contrast to natural streams where annual temperature deviations might approach both lower and upper incipient lethal levels, the relatively constant water temperatures in many trout hatcheries would not provide selective pressures favoring individuals with low or high temperature tolerances. Vincent (1960) reared domestic and wild brook trout under identical conditions and showed that wild fish had a higher upper temperature tolerance than domestic ones; this provided strong evidence of a genetic basis for differences in upper temperature tolerance between strains. In the absence of more definitive studies, we tentatively conclude that differences in upper temperature tolerance of wild and domestic strains of trout results from fundamental genetic differences.
Acknowledgments
This research was supported by the Pennsylvania Fish and Boat Commission. We thank Rickalon Hoopes, William Kennedy, Paul Martis, Thomas Bender, and the staff of the Benner Spring Fish Culture Station for providing facilities, broodstock histories, and fish care. Thomas Beitinger's review of the manuscript is greatly appreciated.




