Growth and Long-Range Dispersal by Wild Subyearling Spring and Summer Chinook Salmon in the Snake River Basin
Abstract
Wild juvenile spring and summer chinook salmon Oncorhynchus tshawytscha typically pass downstream in the Snake River as yearling smolts when migrating seaward. A small number of these fish disperse downstream from natal streams into the Snake River as subyearlings and then continue moving seaward. We estimated that the naturally produced subyearling spring and summer chinook salmon that dispersed into the Snake River in 1993, 1994, and 1997 originated from both wild (67%) and naturalized hatchery (33%) stocks. These fish were large in terms of mean fork length (range, 78–87 mm) during shoreline rearing. While moving seaward, they grew rapidly (range of means, 1.0–1.3 mm/d) to yearling smolt fork lengths (range of means, 122–128 mm). The estimated dispersal distances from natal streams to the first two dams encountered during seaward movement ranged from 172 to 810 km. Although we could not demonstrate seawater entry, we believe that the wild spring and summer chinook salmon had met the growth and size criteria for successful smoltification. We suggest that rapid growth to yearling smolt size reflects comparatively high growth opportunity in the Snake River and that this helps to explain why wild spring and summer chinook salmon that disperse into the Snake River migrate seaward as subyearlings while those that rear in presumably less productive natal streams migrate seaward as yearlings.
Introduction
In the Snake River basin, wild spring and summer (hereafter, spring–summer) chinook salmon Oncorhynchus tshawytscha typically have a stream-type (Healey 1991) life history. Adults enter the mouth of the Snake River in spring and summer (hence the spring-run and summer-run designations), then spawn during late summer in low-order streams in the Salmon, Imnaha, Grande Ronde, and Clearwater river subbasins (Figure 1). Fry emerge primarily from late January through early May (Howell et al. 1984). Wild subyearling spring–summer chinook salmon in the Snake River subbasin typically rear during summer in low-order streams, overwinter in higher-order streams, and then begin seaward migration the following spring as yearling smolts 80–120 mm in length (e.g., Chapman and Bjornn 1969; Bjornn 1971). A small number of wild subyearling spring–summer chinook salmon disperse long distances into the main-stem Snake River and then continue to move downstream at least as far as Lower Granite Dam (Marshall et al. 2000) at river kilometer (rkm; measured from the mouth of the Snake River) 173 (Figure 1).
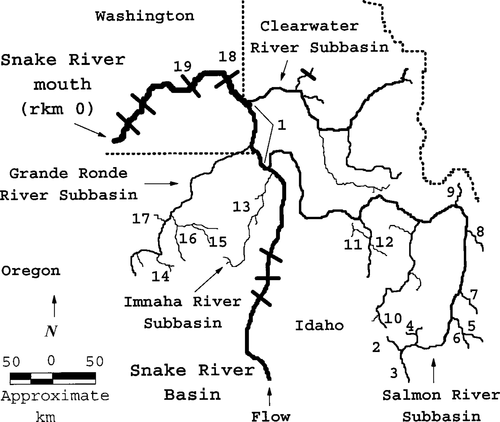
The Snake River basin, including the beach-seining area, several subbasin tributaries where wild spring and summer chinook salmon spawn, and Lower Granite and Little Goose dams, where the PIT-tagged fish were recaptured. The locations of the dams and the sites where genetic baseline samples were collected (Waples et al. 1993; Marshall 1996) are as follows, with the distances from the mouth of the Snake River (rkm 0) given in parentheses: 1 = seining area (224–291); 2 = Valley Creek (923); 3 = Upper Salmon River (920); 4 = West Fork Yankee Fork Creek (905); 5 = Herd Creek (869); 6 = East Fork Salmon River (862); 7 = Pahsimeroi River (792); 8 = Lemhi River (719); 9 = North Fork Salmon River (648); 10 = Marsh Creek (813); 11 = Secesh River (604); 12 = Johnson Creek (602); 13 = Imnaha River (345); 14 = Catherine Creek (548); 15 = Lostine River (426); 16 = Minam River (418); 17 = Lookingglass Creek (408); 18 = Lower Granite Dam (173); and 19 = Little Goose Dam (113)
Connor et al. (2001) compared the early life history attributes of wild subyearling fall and spring–summer chinook salmon recaptured after passing Lower Granite Dam. Wild Snake River fall chinook salmon have an ocean-type (Healey 1991) life history. Adults spawn during the fall in the free-flowing Snake River upstream of Lower Granite Reservoir (Figure 1). Fry emerge in the spring, grow rapidly (0.7–1.1 mm/d), and as subyearling smolts (112–151 mm) pass Lower Granite Dam during late spring and summer (W. P. Connor, unpublished data). There is much overlap in the timing of shoreline rearing, fork length, and passage timing at Lower Granite Dam between the wild subyearling fall and spring–summer chinook salmon (Connor et al. 2001). Wild subyearling fall and spring–summer chinook salmon passing Lower Granite Dam are also morphologically indistinguishable, both having the deep robust bodies indicative of productive rearing habitat (Tiffan et al. 2000).
In this paper, we expand on the findings of Marshall et al. (2000), Tiffan et al. (2000), and Connor et al. (2001) by reporting the likely genetic origins, fork lengths, growth rates, and estimated dispersal distances of wild subyearling spring–summer chinook salmon that were captured and tagged in the Snake River and then recaptured passing Lower Granite Dam in 1993 and 1994 or Little Goose Dam (rkm 113; Figure 1) in 1997.
Methods
Data collection and aging
We beach-seined wild subyearling chinook salmon along the free-flowing Snake River upstream of Lower Granite Reservoir at stations located between rkm 224 and rkm 291 (Figure 1) from April to July (Connor et al. 1998). Sites were characterized by relatively low-velocity pockets of water adjacent to cobble or sandy shorelines that sloped gradually into the river channel. Wild subyearling chinook salmon of at least 60 mm (fork length) were tagged with passive integrated transponder (PIT) tags (Prentice et al. 1990) and then released at the collections sites after a 15-min recovery period to resume rearing and downstream movement (Connor et al. 1998). We recaptured some of the PIT-tagged fish at Lower Granite Dam in 1993 and 1994 and at Little Goose Dam in 1997 (Connor et al. 2001). We measured the fork length and then sampled the scales and the body muscle, heart, liver, and eye tissues of each recaptured fish (Marshall et al. 2000). We used the scales to confirm that fish were subyearlings (Koo 1967).
Genetic analyses
We identified spring lineage juveniles (the spring and summer runs have a common genetic lineage) in the samples of recaptured chinook salmon by genetic mixture analysis using maximum likelihood estimation procedures (Marshall et al. 2000). The baseline allozyme allele frequency data used by Marshall et al. (2000) for maximum likelihood analyses included only samples from Salmon and Clearwater river stocks because the data from other Snake River basin stocks did not include some loci and alleles that are useful for discriminating between fall and spring lineages (Marshall et al. 2000). Therefore, we created a new baseline data set to estimate the stock composition of the spring lineage subyearlings. We assembled allele frequency data for wild or naturally spawning spring–summer chinook salmon stocks from the Salmon, Imnaha, and Grande Ronde river subbasins (Table 1) because juveniles had been collected and PIT-tagged in the Snake River downstream of these drainages. We used the Rapid River Hatchery stock sampled at Lookingglass Hatchery (Waples et al. 1993) to assess the prevalence of naturalized hatchery-origin spring chinook salmon in our samples of recaptured PIT-tagged fish. Marked Rapid River Hatchery juveniles (mostly yearlings) have been outplanted in many streams in the Snake River basin (Matthews and Waples 1991) to establish natural production. For example, a mixture of hatchery and naturalized adults of Rapid River Hatchery stock origin spawn in the lower reaches of Lookingglass Creek, Oregon (Figure 1), and thousands of fry can disperse during the spring from Lookingglass Creek to unknown destinations downstream (M. McClean, Oregon Department of Fish and Wildlife, unpublished data).
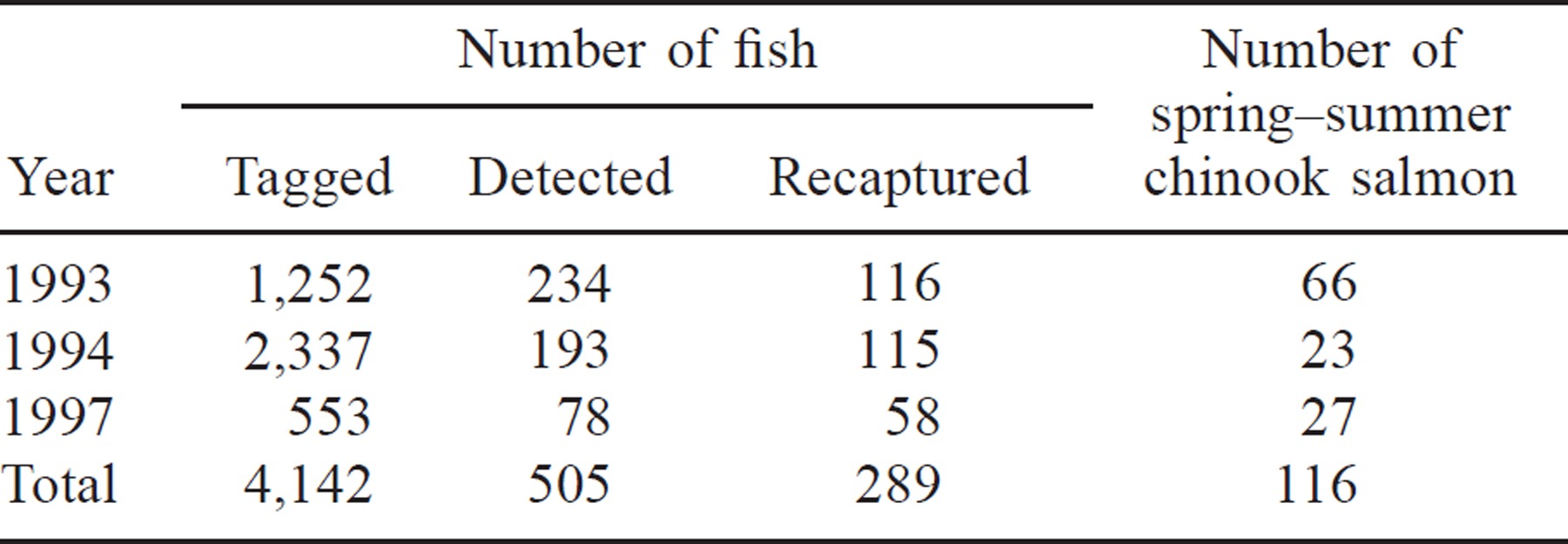
We used data for 23 allozyme loci from each baseline sample, namely, sAAT-1,2*, sAAT-3*, sAAT-4*, mAAT-1*, ADA-1*, sAH*, FDHG*, GR*, sIDHP-1*, sIDHP-2*, LDH-B2*, LDH-C*, sMDH-B1,2*, mMDH-2*, sMEP-1*, MPI*, PEPA*, PEPB-1*, PEPD-2*, PEP-LT*, PGK-2*, sSOD-1*, and TPI-4*. The genotypes of the recaptured fish at these 23 loci were available from the original electrophoretic analysis (Marshall et al. 2000). We used the maximum likelihood estimation computer program of Milner et al. (1983) as modified by Millar (1987) to estimate the contributions from each baseline population. We computed grouped contribution estimates from the Salmon River and the Imnaha and Grande Ronde river subbasin stocks by summing individual population estimates and recalculating the variances for both groups. See Appendix 1 for the mean allele frequencies of these two population groupings and those of the Rapid River Hatchery stock. We report the contributions of the latter separately because fish of this origin cannot be attributed accurately to one drainage due to the widespread distribution of this stock.
Growth and dispersal
We calculated absolute growth rate for each PIT-tagged wild subyearling spring–summer chinook salmon that was recaptured at Lower Granite or Little Goose dam as the fork length at recapture minus the fork length at initial capture divided by the number of days between initial capture and recapture. We estimated the range of distances these fish may have dispersed from natal tributaries to the dams by using the distance information in Figure 1. For example, the dispersal distance between Lookingglass Creek, Oregon, and Lower Granite Dam is 235 km (408 − 173 km).
Results
We inserted PIT tags into 4,142 wild subyearling chinook salmon during 1993, 1994, and 1997 (Table 2). The total number of tagged wild subyearling chinook salmon detected at Lower Granite or Little Goose dam was 505, of which 289 were recaptured and genetically analyzed (Table 2). The total number of spring–summer chinook salmon that were genetically identified was 116 (Table 2).
We estimated that the recaptured fish originated from a variety of stocks, with the highest contribution coming from the Imnaha and Grande Ronde river subbasin group (48%; Table 3). We estimated that one-third of the fish recaptured at the dams were derived from naturalized Rapid River Hatchery stock. Juveniles from Salmon River subbasin stocks were estimated to comprise the smallest percentage (19%) of PIT-tagged fish recaptured at the dams.

The wild subyearling spring–summer chinook salmon were initially captured, PIT-tagged, and released along the Snake River during spring and the first few days of summer (Table 4). Mean fork length at this time ranged from 78.3 to 87.2 mm (Table 4).
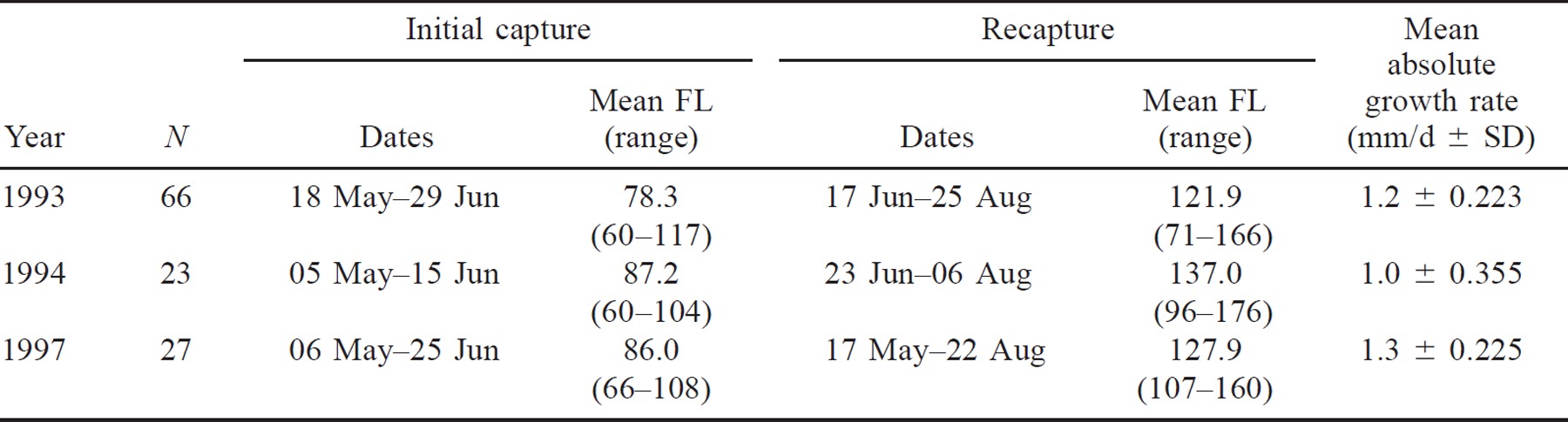
The PIT-tagged fish passed Lower Granite or Little Goose dams from late spring through midsummer (Table 4). During the time between release and recapture at the dams, the growth rate averaged 1.0–1.3 mm/d and mean fork lengths at the time of recapture ranged from 121.9 to 137.0 mm (Table 4). The estimated dispersal distances from spring and summer chinook salmon rearing areas to Lower Granite Dam ranged from 172 to 750 km; those to Little Goose Dam ranged from 232 to 810 km.
Discussion
We found that some wild subyearling spring–summer chinook salmon disperse from natal streams in the Salmon, Imnaha, and Grande Ronde river subbasins into the Snake River, where they grow and continue seaward movement. We did not collect data to establish causal linkages between our findings and the many factors that affect the dispersal, growth, and age at seaward migration of juvenile chinook salmon. Volumes of data are being collected on Snake River spring–summer chinook salmon that spawn in the wild to monitor and evaluate Endangered Species Act recovery measures. These data could be used to test the ideas in the following discussion and to determine whether long-range dispersal behavior has any implications for the management of wild Snake River spring–summer chinook salmon listed for protection under the Endangered Species Act (NMFS 1992).
The wild subyearling spring–summer chinook salmon that dispersed into the shoreline habitat of the Snake River were large for the time of year they were captured. Wild spring–summer chinook salmon that rear in the Salmon, Imnaha, and Grande Ronde river subbasins do not usually grow to 60–117 mm until midsummer or fall, and some do not reach these lengths until the next spring (Bjornn 1971, 1978; Horner 1978; Howell et al. 1984; Achord et al. 1996). Fork-length-dependent dispersal (Bradford and Taylor 1997) and accelerated growth are two possible explanations for the relatively large size of wild subyearling spring–summer chinook salmon that disperse into the Snake River. Bradford and Taylor (1997) found that dispersing stream-type fry were only 1 mm longer than nondispersing fry. Therefore, accelerated growth by dispersing fish is the likely explanation for the large size of the wild subyearling spring–summer chinook salmon in the Snake River.
The wild spring–summer chinook salmon captured in the Snake River continued to move seaward after being PIT-tagged, and they were eventually recaptured from 172 to 810 km downstream from the known rearing areas in the Salmon, Imnaha, and Grande Ronde river subbasins. To our knowledge, these dispersal distances are the longest reported in the peer-reviewed literature for wild spring–summer chinook salmon subyearlings. For comparison, the wild stream-type chinook salmon fry that Bradford and Taylor (1997) studied in the Fraser River, British Columbia, moved up to 100 km downstream from natal streams. Dispersing 100 km downstream might allow fry to utilize all available rearing habitat (Bradford and Taylor 1997). Dispersing even longer distances could provide evidence of actual seaward migration.
Although it cannot be confirmed that the wild subyearling spring–summer chinook salmon that we recaptured at Lower Granite and Little Goose dams would have completed a seaward migration if left in the river, we believe that they met the growth and size criteria required for survival in seawater. The wild chinook salmon that we studied grew rapidly (1.0–1.3 mm/d) in the Snake River by comparison with subyearling spring chinook salmon in presumably more productive estuarine and ocean environments (0.4–1.3 mm/d; Healey 1980; Buckman and Ewing 1982; Kjelson et al. 1982). Rapid growth allowed these fish to reach yearling smolt size (80–120 mm; Bjornn 1971) by spring and early summer, and it occurred during the critical spring time period associated with successful smoltification (Dickhoff et al. 1997; Beckman and Dickhoff 1998).
In conclusion, we propose that wild subyearling spring–summer chinook salmon that disperse into the Snake River grow more rapidly than the members of their cohort that remain in natal streams. Rapid growth sustained during critical time periods in the spring and early summer reflects comparatively high growth opportunity (Metcalfe and Thorpe 1990; Taylor 1990), and it helps to explain why some wild subyearling spring–summer chinook salmon that disperse from presumably less productive natal streams migrate seaward as subyearlings rather than as yearlings.
Acknowledgments
Many employees of the U.S. Fish and Wildlife Service, the Washington Department of Fish and Wildlife, and the U.S. Geological Survey assisted during the study. We thank the staff at the Pacific States Marine Fisheries Commission for managing the PIT-tag database. The hardware and software used to recapture smolts at the dams was developed and tested by S. Downing and E. Prentice. R. Waples provided genetic data. D. Bennett, J. Congleton, K. Steinhorst, and M. Faler, and three anonymous reviewers made suggestions that improved paper. The U.S. Fish and Wildlife Service Lower Snake River Compensation Plan and the rate payers of the Bonneville Power Administration (contract number DE-AI79-91BP21708) funded the study. D. Docherty was the contracting officer.
Appendix: Allozyme Data