Lake Trout Reproduction in Lake Champlain
Abstract
Native lake trout Salvelinus namaycush were driven to extirpation in Lake Champlain in the early 1900s. Possible causes include overharvest, predation on adults by sea lamprey Petromyzon marinus, and predation on fry by rainbow smelt Osmerus mordax. Efforts to restore a lake trout fishery began in 1972 when a coordinated stocking program was initiated. Attempts to control sea lamprey populations began in 1990. Despite these management actions, reproduction by stocked fish has not resulted in large, naturally produced year-classes. This is the first formal study to quantitatively assess the level of natural reproduction by lake trout in Lake Champlain. In 2000–2002, we located 14 potential lake trout spawning sites and evaluated the habitat characteristics and level of spawning activity at each site. Passive egg collectors revealed that eggs were deposited at 8 of 14 sites, egg abundance ranging from 1.9 to 9,623 eggs/m2. In 2001 and 2002, lake trout fry were collected in emergent fry traps at three of five sites; catch per unit effort ranged from 0.08 to 2.38 fry·trap−1·d−1. We were unable to collect naturally produced juvenile lake trout through bottom trawling. We also examined adult lake trout size structure and abundance data collected annually by state agencies to determine whether there was a trend in the percentage of unmarked lake trout in the population. The percentage of unclipped lake trout in Lake Champlain decreased steadily from 1982 (7.4%) to 1988 (1.7%), was variable from 1989 to 1991, increased from 1992 (2.6%) to a maximum in 2000 (10.4%), and then decreased to 5.7% in 2001. The high levels of egg and fry abundance, the failure to collect lake-produced juveniles, and the low percentage of unclipped adult fish all suggest that a recruitment bottleneck is present during the postemergent fry life stage.
Introduction
Lake Champlain supported self-sustaining populations of lake trout Salvelinus namaycush prior to and during the 19th century, when the Lake Champlain valley was settled and developed (Plosila and Anderson 1985). A commercial fishery was not present, though lake trout populations were large enough to allow seine harvests of spawning stocks during the fall. In the mid to late 1800s, lake trout populations began to decline in Lake Champlain and became extinct by 1900 (Plosila and Anderson 1985). No data were collected on population characteristics or abundance prior to and during the period of decline.
Lake trout populations also underwent catastrophic collapses throughout most of the Great Lakes during the 19th and 20th centuries (Hansen 1999), but in contrast to these declines, the reasons for the population crash in Lake Champlain are poorly understood. It is generally accepted that a combination of overharvest, predation by sea lampreys Petromyzon marinus, and cultural eutrophication caused lake trout populations to crash throughout the Great Lakes basin (Eschmeyer 1957; Eshenroder 1992; Cornelius et al. 1995; Elrod et al. 1995; Hansen et al. 1995). While possible explanations for the decline of lake trout in Lake Champlain include overharvest (Plosila and Anderson 1985), predation by rainbow smelt Osmerus mordax (Halnon 1963), and predation by sea lampreys, historical data do not exist to support or refute these explanations. A commercial lake trout fishery did not exist in Lake Champlain, and subsistence fishing in the 1800s probably had no significant impact on lake trout populations considering there were sparse human populations and the fishing methods employed (seine) were inefficient at the depths at which lake trout are generally found. Rainbow smelt are native to Lake Champlain and coevolved with lake trout, suggesting that rainbow smelt predation on lake trout was not likely to have caused the lake trout population to decline. Sea lamprey populations were described as common and abundant in Lake Champlain in the early to mid-1800s (Halnon 1963). It is unclear whether sea lampreys are native or exotic to Lake Champlain, and this is currently a topic of substantial debate. If sea lampreys are native, they would have entered Lake Champlain approximately 10,000 years ago and have coexisted with lake trout. If they are exotic to Lake Champlain, they would have entered in the mid to late 1800s (after the construction of the Champlain and Chambly canals) and may have been a significant factor in lake trout population declines. However, anecdotal evidence suggests that sea lamprey populations were at low levels in the late 1800s when lake trout populations were declining because forestry practices made tributaries unsuitable for sea lamprey reproduction (B. Chipman, Vermont Department of Fish and Wildlife, personal communication).
The first attempts to restore lake trout in Lake Champlain took place in the late 19th century. Sporadic stockings were unsuccessful in reestablishing naturally reproducing populations (Plosila and Anderson 1985). In the late 1950s and the 1960s, the New York State Department of Environmental Conservation (NYSDEC) and the Vermont Department of Fish and Wildlife (VTDFW) stocked limited numbers of lake trout. This stocking attempt was successful in developing a small lake trout fishery, but it failed to produce a self-sustaining lake trout population. Stocking levels increased in 1973 when the NYDEC, the VTDFW, and the U.S. Fish and Wildlife Service (USFWS) signed an agreement to form the Lake Champlain Fish and Wildlife Management Cooperative (LCFWMC). A primary goal of the cooperative was to reestablish a lake trout fishery in the lake. In the absence of information about historic stock characteristics, the goals of the restocking program focused on reestablishing a fishery rather than restoring a lake trout population. The specific objective developed by the cooperative in 1977 was to “reestablish a lake trout fishery by 1985 that will annually provide at least 45,000 additional man-days of fishing with an approximate yield of 18,000 lake trout averaging 5 lb each” (Fisheries Technical Committee 1977). Since 1973, nearly 5 million lake trout have been stocked; annual stocking rates have been variable and range from 39,000–271,863 yearling equivalents (5 fall fingerlings = 1 spring yearling; Fisheries Technical Committee 1999; Figure 1). Stocking rates were decreased by approximately half in 1995 after bioenergetics modeling suggested that the higher stocking levels, coupled with increased survival from the experimental sea lamprey control program, could potentially cause the rainbow smelt forage base to crash (LaBar 1993). Since this reduction, annual stocking rates have stabilized between 68,000 and 90,000 yearlings. Several different lake trout strains have been stocked, including the Adirondack (Raquette Lake and Lake George), Finger Lakes (Seneca Lake), Lake Michigan (Green Lake), Manitoba (Clearwater), Lake Superior (Marquette), Maine (Allagash Lake), and Jenny Lake (Wyoming; Fisheries Technical Committee 1999). More recently, a “Lake Champlain” strain (progeny of feral lake trout from Lake Champlain) has been produced and stocked (Figure 1). Since 1990, only the Seneca Lake and Lake Champlain strains have been stocked in the system. These strains were selected because the Seneca Lake strain may be more resistant to sea lamprey attacks than other strains (Swink and Hanson 1986), and the Lake Champlain strain was primarily produced from Seneca Lake strain parents.
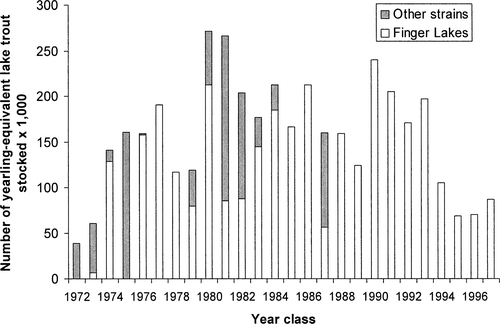
Numbers of yearling-equivalent lake trout stocked into Lake Champlain by year-class and strain, 1972–1997. “Finger Lakes” includes the Seneca Lake strain and composite strains of progeny from feral lake trout in Lakes Ontario and Champlain, which were assumed to be largely of Seneca Lake origin. “Other strains” include fish from Jenny Lake, Lake Michigan, Lake Superior (Marquette), Manitoba (Clearwater), Adirondack (Raquette Lake and Lake George), and Maine (Allagash Lake)
A second strategy to increase lake trout populations involved controlling populations of sea lampreys. An 8-year experimental sea lamprey control program was initiated in 1990 and applied larval lampricides to 13 tributary systems and 5 deltas (Marsden et al. 2003). The program reduced the wounding rates (types AI–AIII) of stocked lake trout and consequently increased survival. Prior to sea lamprey control, wounding rates were greater than 50%; during the period of experimental sea lamprey control, wounding rates fluctuated between 30% and 50% (Marsden et al. 2003). Sea lamprey control increased the mean survival rate of age-3–4 lake trout from 0.35 to 0.43 and that of age-5–9 lake trout from 0.51 to 0.59; this mortality rate includes the fisheries exploitation rate, which varied from 0.11 to 0.14 between 1991 and 1997 (Fisheries Technical Committee 1999; Marsden et al. 2003). Stocked lake trout have established a recreational fishery that has exceeded the management goal set by LCFWMC in 1977. In summer gill netting conducted by LCFWMC from 1982 to 1997 (see Methods), catch per unit effort (CPUE; fish/net) ranged from 3 to 11, and multiple year-classes of sexually mature (>age-5) fish were collected each year (Fisheries Technical Committee 1999). Since 1991, repeat spawners (≥age-7) have represented between 19% and 47% of all lake trout sampled. However, reproduction by these fish has not contributed significantly to the adult population.
It is unlikely that the same impediments to lake trout rehabilitation in the Great Lakes are occurring in Lake Champlain. Adult stock size appears to be adequate; Selgeby et al. (1995) developed stock size criteria for the Great Lakes and determined that areas with recruitment of age-1 and older lake trout had a CPUE in fall gill nets of 17–135 fish/305 m. In Lake Champlain, sampling with gill nets was conducted in summer, when lake trout are more highly dispersed; nevertheless, the equivalent catch was 7.5–27.5 fish/305 m of gill net, partially within the range of naturally reproducing populations analyzed by Selgeby et al (1995). The failure of lake trout to reproduce in the Great Lakes has been attributed to the accumulation of contaminants by lake trout (Mac and Edsall 1991). Madenjian et al. (2001) reviewed research on the role that contaminants may have played on lake trout reproductive success and concluded that contaminants have had very little effect on lake trout recruitment in Lake Michigan. Contaminant concentrations in Lake Champlain lake trout (R. Langdon, Vermont Department of Environmental Conservation, unpublished data) are lower than recent concentrations found in Lake Michigan lake trout (Madenjian et al. 2001) and therefore are unlikely to inhibit the natural recruitment of lake trout in Lake Champlain. The lack of genetic diversity in stocked lake trout populations is also considered an important constraint to lake trout rehabilitation in the Great Lakes (Burnham-Curtis et al. 1995). Natural reproduction may be enhanced when genetic diversity is maximized by stocking multiple strains and when appropriate strains are matched with stocking locations (Burnham-Curtis et al. 1995; Perkins et al. 1995). In the 1970s and 1980s, seven different lake trout strains were stocked into Lake Champlain, including strains from the Great Lakes and Finger Lakes of New York. The Seneca Lake strain, which was stocked in the highest frequency, has shown consistent reproductive success lakewide in Lake Ontario (Grewe et al. 1994). Early mortality syndrome (EMS) is another important source of lake trout mortality in the Great Lakes that is absent in Lake Champlain. Early mortality syndrome is linked to a diet high in exotic alewife Alosa pseudoharengus that contain high levels of thiaminase (Fitzsimons 1995a). Alewives are not present in Lake Champlain and the main forage fish, rainbow smelt, contain approximately one-half the level of thiaminase found in alewives (J. Fitzsimons, Canada Centre for Inland Waters, personal communication). Native lake trout in Lake Champlain were self-sustaining with a diet of native smelt, as there are few other forage species in the lake; the decline of Great Lakes lake trout did not occur for almost six decades after the introduction of smelt into the lakes in the 1920s and 1930s (Christie 1974). In the spring of 2001, approximately 150 lake trout sac fry were collected from the in Grand Isle breakwall and held at the Ed Weed Fish Culture Station in Grand Isle until the yolk sac was fully absorbed; fry survival was greater than 90% and no signs of EMS were detected (D. Kelsey, VTDFW, personal communication). Jones et al. (1995) developed a quantitative model of lake trout egg and fry predation and concluded that predation mortality could block natural recruitment, especially in systems where exotic predators have become established. To date, Lake Champlain has had no additions of known lake trout egg or fry predators.
Despite the efforts of managers to increase lake trout populations in Lake Champlain, lake trout have not reestablished self-sustaining populations. Since the mid-1980s, adult lake trout in Lake Champlain have exhibited typical spawning behavior by aggregating during autumn at sites with appropriate spawning substrate (i.e., clean rock substrate with deep interstitial spaces; B. Chipman, VTDFW, personal communication). However, prior to this study there was no attempt to confirm lake trout natural reproduction at these sites. The goal of this study was to determine the level of lake trout natural reproduction in Lake Champlain. The objectives were to (1) identify potential lake trout spawning sites, (2) intensively assess lake trout reproductive success (egg deposition, fry hatch, and fry survival) at identified sites, and (3) evaluate the contribution of unclipped lake trout to the juvenile and adult population.
Methods
Lake description
Lake Champlain forms the boundary between Vermont and New York and extends north into the Canadian province of Quebec (Figure 2). The lake is 193 km long, and has a maximum width of 19 km and a surface area of 1,127 km2 (Lake Champlain Basin Program 1999). The mean depth is 19.5 m and the maximum depth is approximately 120 m; the main basin of the lake is primarily meso- to oligotrophic (Lake Champlain Basin Program 1999).
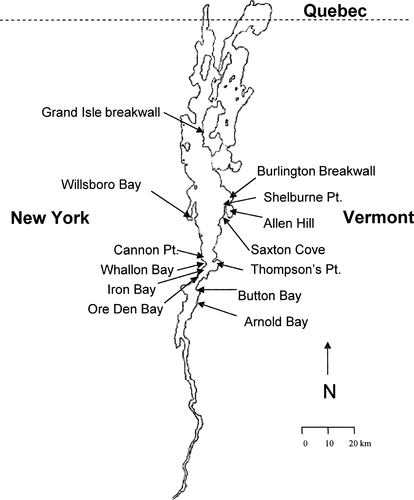
Locations of areas examined for lake trout spawning in Lake Champlain.
Assessment of unclipped lake trout
Annual assessment surveys for adult lake trout have been conducted since 1982 by VTDFW and NYDEC. From 1982–1997, the surveys were conducted during June, July, and August using nylon multifilament gill nets 122 m long and 1.8 m deep. Gill nets were composed of eight 15-m panels of different stretch mesh sizes (64, 76, 89, 102, 114, 127, 140, and 152 mm) and were fished for 24 h. Age-4 and older (>400 mm) lake trout are fully selected to the gear while age-3 fish (<400 mm) are only partially selected (Fisheries Technical Committee 1999). Beginning in 1989, state agencies started fall electrofishing surveys to provide a second index of population abundance, size, and age structure. In 1997, agencies converted from summer gill netting to electrofishing only. Because all lake trout stocked into Lake Champlain are marked with a fin clip, an individual fish is assumed to be naturally produced if it has no fin clips. However, unclipped fish may also include those that were mistakenly not clipped prior to stocking or that regenerated the clipped fin and were therefore not recognized as having been clipped. The surveys provided an extensive data set to evaluate the proportion of unclipped age-3 and older lake trout and changes in abundance, age, and size structure through time.
Spawning site surveys
In the summers of 2000 and 2001, we located potential spawning sites using bathymetric charts, observations of shoreline geology, scuba observations, remotely operated video, and discussions with Lake Champlain fisheries biologists. We were seeking sites with habitat characteristics believed to be important for lake trout spawning, including cobble or boulder substrate, deep (>10 cm) interstitial spaces, the presence of low amounts of silt, and adjacency to a steep slope (Fitzsimons 1995b; Marsden et al. 1995). Cobble is defined as particles with diameters of 25.7–99.9 cm; particles larger than cobbles are defined as boulders, as used in previous examinations of lake trout spawning habitat (Marsden et al. 1995). Spawning habitat quality and lake trout spawning levels were evaluated at selected sites. Variables measured to evaluate spawning habitat quality included reef depth, reef slope, substrate size, and interstitial depth. The approximate area of spawning sites was estimated using a combination of measurements of reef dimensions conducted by divers with a 100-m measuring tape, and visual estimates of small areas by divers. Divers made visual observations of the relative abundance of macrophytes and zebra mussels Dreissena polymorpha and estimated the abundance of egg and fry predators (such as slimy sculpins Cottus cognatus and crayfish Orconectes spp.) as the frequency of sightings of each species observed while burying egg bags or surveying a reef. These observations provided a crude estimate of relative abundance.
Lake trout spawning activity assessment
Once potential spawning sites were identified, we assessed egg deposition in fall of 2000 and 2001 using quantitative and qualitative passive egg collectors. The qualitative egg collection gear consisted of 15 egg nets (Horns et al. 1989) and 15 egg traps (Marsden et al. 1991) attached alternately 1 m apart to a 30-m line with anchors and buoys at each end. The gangs of traps and nets were deployed in early fall just prior to spawning and checked for the presence of eggs weekly until spawning was finished. The quantitative egg collectors (bags) consisted of a 45.7-cm-deep cloth bag (3-mm mesh) attached to a 29.8-cm-diameter polyvinyl chloride (PVC) ring (Perkins and Krueger 1994). Scuba divers buried the bags by excavating a 40–50-cm-deep pit, placing an individual bag into a pit, and backfilling the bag with the removed substrate. Egg bags were buried prior to spawning (August–October) and were retrieved when spawning was believed to have finished (late November–early December). During the 2000 spawning season, five sites were sampled using one gang of the qualitative egg collectors (15 traps and 15 nets) per site. Additionally, a remotely operated vehicle (ROV) equipped with a suction sampler was used in a related study to explore for lake trout spawning in deep water. In 2001, 10 sites were sampled using quantitative egg collectors, and two sites were sampled using qualitative egg collectors. Eggs collected from each site were counted and preserved in Stockard's solution. Empty egg shells (chorions) also supplied evidence of the presence of eggs, and were counted and included with egg totals.
A subset of sites where lake trout deposited eggs were sampled for fry in the spring of 2001 and 2002 using two types of surface-deployed qualitative emergent fry traps. A rigid steel trap (Marsden et al. 1988) and a similarly designed nylon mesh trap (Chotkowski et al. 2002) were deployed from ice-out (April) through mid-June. In 2001, fry sampling was conducted at the Grand Isle breakwall, Whallon Bay, Ore Den Bay, and Arnold Bay. In 2002, fry sampling was conducted at the Grand Isle breakwall, Saxton Cove, Whallon Bay, and Arnold Bay. Each site was sampled with 7–20 traps that were checked weekly for the presence of fry.
Juvenile lake trout sampling
In summer of 2001, we used a bottom trawl (7.62-m headrope, 6.35-mm stretched-mesh cod end) to sample for yearling and older lake trout at Shelburne, Whallon, and Willsboro bays. Sampling occurred on May 18 and July 18, 2001, at Shelburne Bay, on July 18, 2001, at Whallon Bay, and on August 16, 2001, at Willsboro Bay. The depths sampled ranged from 15 to 40 m. The duration of bottom trawling at 2.2 knots was 79 min at Shelburne Bay, 26 min at Whallon Bay, and 70 min at Willsboro Bay. All lake trout captured were examined for fin clips, measured for total length, and then released.
Results
Assessment of Unclipped Lake Trout
The percentage of unclipped lake trout in Lake Champlain decreased steadily from 1982 (7.4%) to 1988 (1.7%), fluctuated from 1989 to 1991, and increased from 1992 (2.6%) to 2000 (10.6%; Figure 3). In 2001, the percentage decreased to 5.7%.
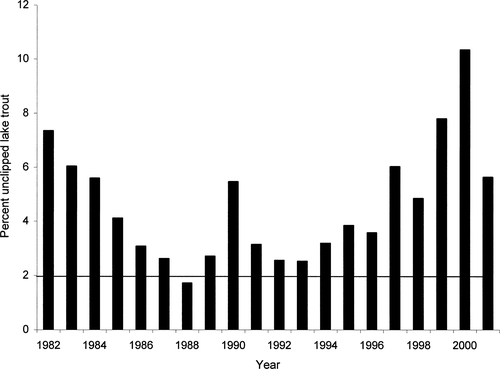
Percentage of unclipped lake trout collected in adult population assessments in Lake Champlain. The line at 2% represents the estimated level of missed fin clips or unidentified hatchery fish, above which recruitment is presumed to be occurring (Schneider et al. 1990; Holey et al. 1995)
Spawning Site Surveys
Since the mid-1980s, New York and Vermont state biologists have observed large aggregations of adult lake trout at two sites (Grand Isle breakwall and Whallon Bay) during the fall, providing indirect evidence of spawning. In 1998 and 1999, preliminary studies (J. E. Marsden, unpublished data) sampled eggs and fry at the Grand Isle breakwall and Whallon Bay, confirming spawning activity at these sites. The Grand Isle breakwall consists of angular cobbles and boulders 13–105 cm in diameter, piled 15–86 cm deep on a 35–60° slope (Table 1). In fall, the water depth above the cobble substrate ranges from 0.3 to 5 m. The area of cobble is approximately 570 m2. Whallon Bay has round substrate composed of natural, rounded cobble and boulder ranging from 17 to 106 cm in diameter with interstitial spaces 4–29 cm deep (Table 1). Whallon Bay is a moderately sloping, north facing shoreline, approximately 64,372 m2 in area, and ranges from 0.3 to 14 m in depth.
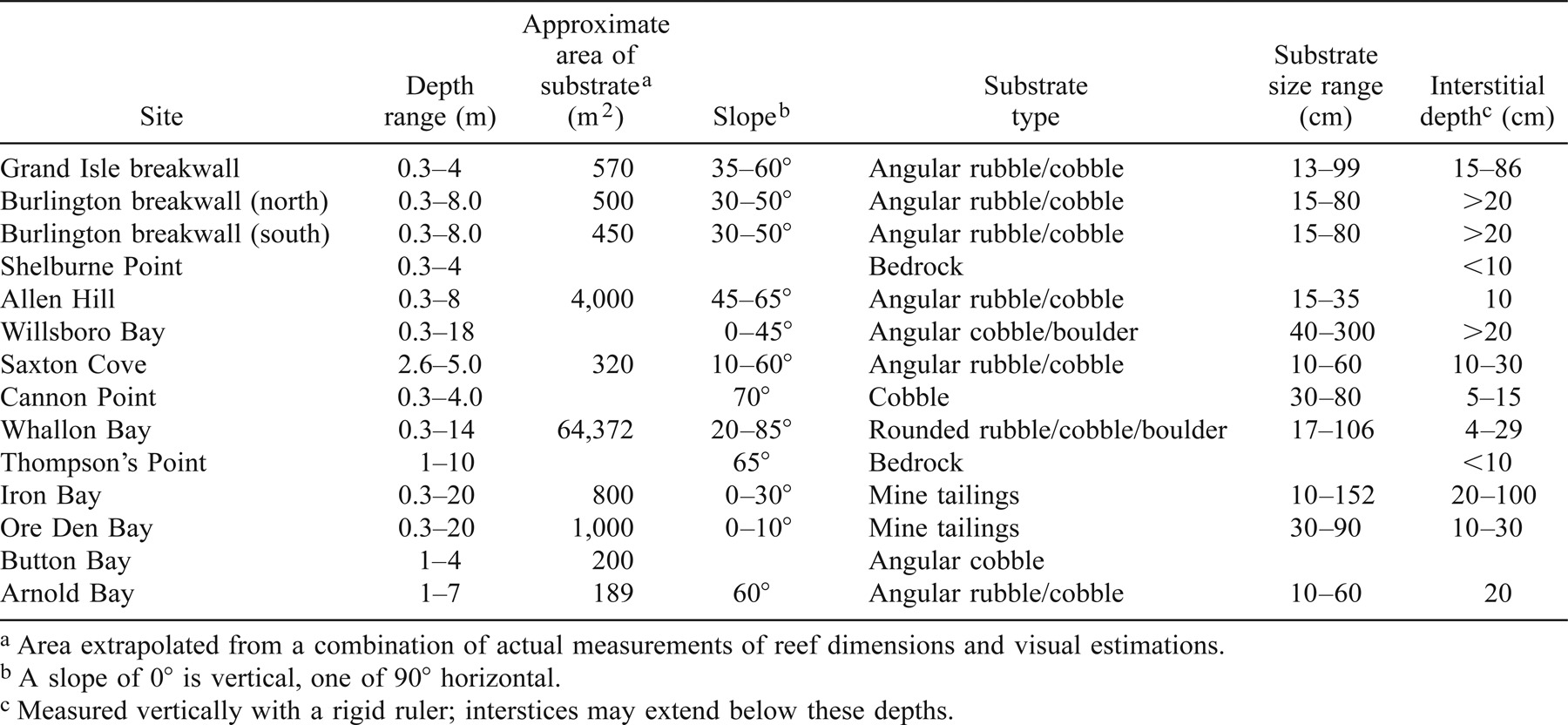
Four sites in 2000 and 8 sites in 2001 were chosen as potential lake trout spawning areas (Figure 2; Table 1). Burlington breakwall (north and south), Willsboro Bay, Saxton Cove, Iron Bay, Ore Den Bay, and Arnold Bay are artificial sites either intentionally created to protect water intake pipes or to construct piers, or unintentionally created through mining or railroad construction. Shelburne Point, Allen Hill, Cannon Point, Thompson's Point, and Button Bay are sites composed of natural rock materials. In total, 14 sites were evaluated for biotic and abiotic habitat characteristics and for assessment of lake trout spawning (Table 1). Macrophytes were sparse or absent at all sites except Iron Bay, Ore Den Bay, and Button Bay. Egg and fry predator abundance was observed to be low at most sites (<5 predators/m2). Zebra mussel abundance was moderate to dense at all sites except the two Burlington breakwall sites and Cannon Point. At moderate densities, zebra mussels covered over 90% of hard surfaces in at least a single layer, and at higher densities they formed colonies several individuals thick over all hard surfaces.
Lake Trout Spawning Activity Assessment
Egg deposition occurred at 8 of 14 sites sampled in 2000 and 2001. In 2000, eggs were collected at four of five sites from November 13 to December 11 (Table 2). Catch per unit effort ranged from 0.002 to 4.08 eggs·trap−1·d−1. Eggs were collected at a rate of 14.5/min from one additional site (Ore Den Bay) using a suction sampler mounted on a remotely operated vehicle. In 2001, eggs were collected from 6 of 12 sites sampled. The mean egg density at sites where eggs were collected was lowest at Iron Bay (1.9 ± 1.3 eggs/m2 [mean ± SE]) and highest at Grand Isle (9,623 ± 1,658 eggs/m2). Chorions comprised less than 12.1% of the collections at any site and was less than 4% at most sites.
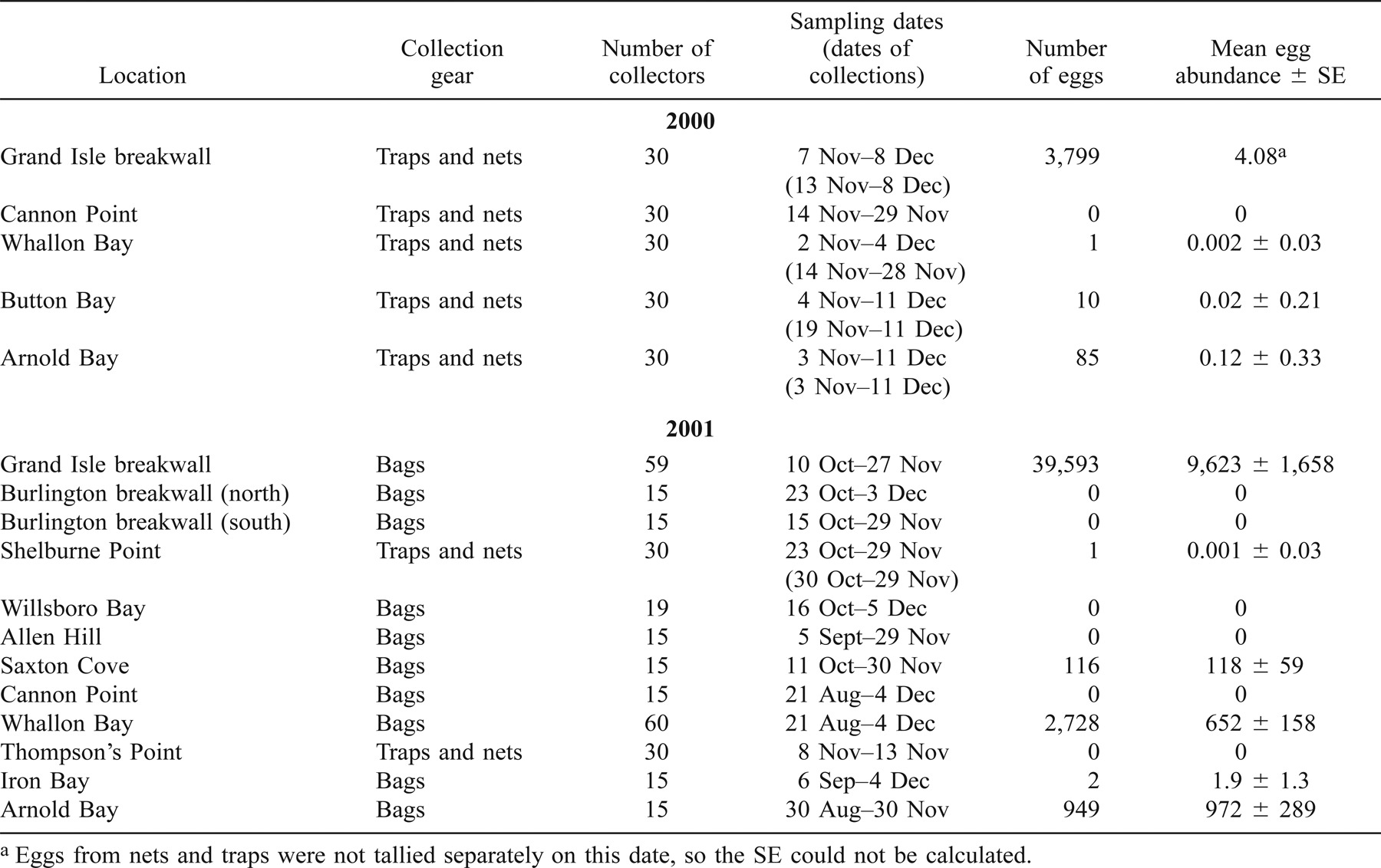
In 2001, fry were caught at three of four sites (Grand Isle breakwall, Whallon Bay, and Arnold Bay) from the beginning of the sampling period to the second week in June (Table 3). Total CPUE (fry·trap−1·d−1) among these three sites was highest at Arnold Bay (2.38), intermediate at the Grand Isle breakwall (0.33), and lowest at Whallon Bay (0.14). Ten fry traps were set at Ore Den Bay and yielded no fry.
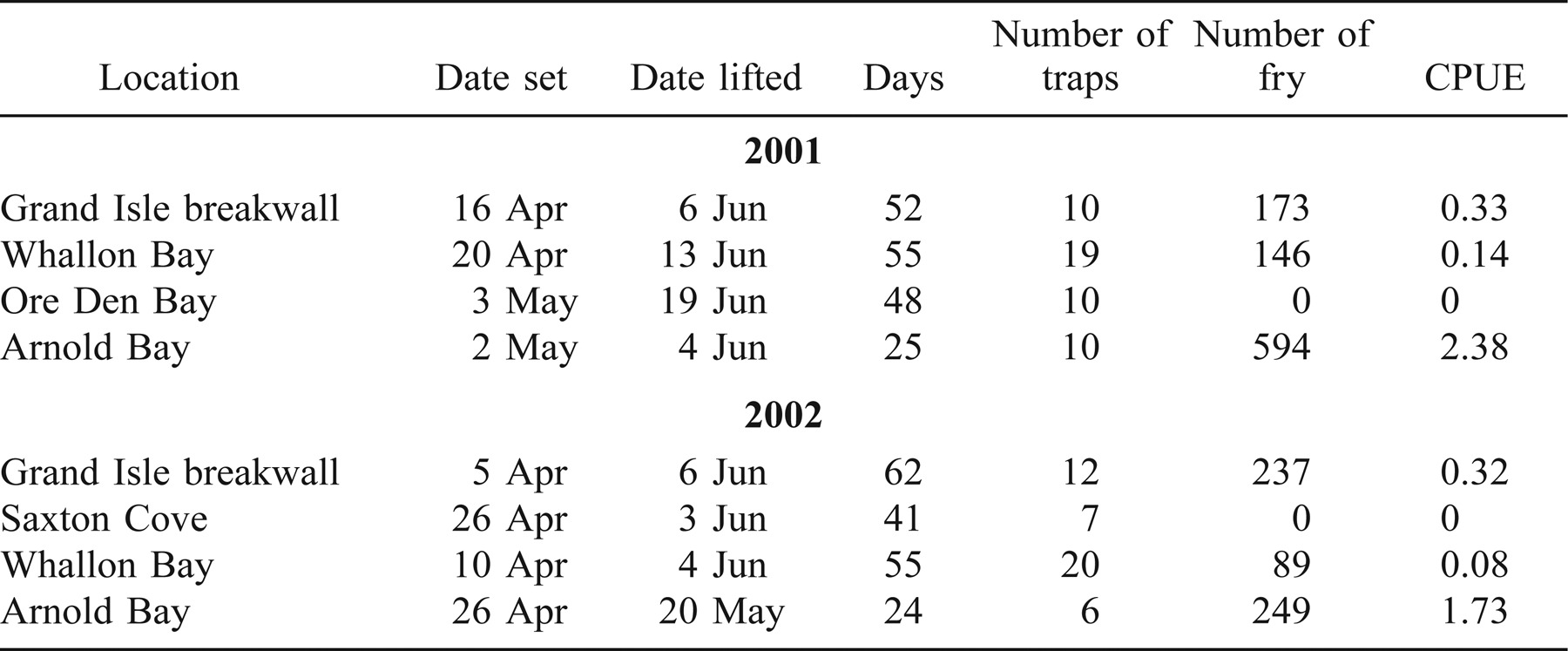
In spring 2002, fry were collected from three of four sites sampled. Fry CPUE remained high at Arnold Bay (1.73), intermediate at the Grand Isle breakwall (0.32), and lowest at Whallon Bay (0.08).
Juvenile Lake Trout Sampling
In 175 min of bottom trawling at 2.2 knots, we collected 11 clipped and zero unclipped lake trout. All 11 fish were collected from Shelburne Bay at a rate of 0.14 fish/min bottom time and ranged from 217 to 396 mm total length. No lake trout were collected from either Whallon Bay or Willsboro Bay.
Discussion
This is the first formal study of lake trout spawning activity in Lake Champlain. Our survey of lake trout spawning habitat revealed the presence of multiple artificial and natural reefs with substrate suitable for egg incubation. With the exception of Willsboro Bay, Iron Bay, and Ore Den Bay (where human-deposited cobble extended down steep slopes to depths up to 20 m), all of the spawning substrate was in shallow water (<8 m). Lake charts and anecdotal information from lake biologists suggest that there are additional sites not examined in the present study that have appropriate lake trout spawning habitat; with the exception of the Grand Isle site, we focused our work only on the middle third of the lake. Based on this information, the availability of spawning habitat does not appear to limit lake trout production.
Lake trout spawned at several sites throughout the main lake, with relatively high rates of egg deposition at three locations compared with the Great Lakes. Lake trout egg deposition in Lake Champlain is an order of magnitude greater than in Parry Sound, Lake Huron, where the lake trout population is considered to be restored based on several criteria (Reid et al. 2001). The maximum CPUE with egg nets and traps was 4.08 in Lake Champlain, whereas the maximum CPUEs from lakes Superior (0.06), Michigan (0.07), Huron (0.46), and Ontario (0.45) were much lower (Schreiner et al. 1995). Extremely high lake trout egg deposition occurred at the Grand Isle breakwall in 2001, where mean egg density (eggs/m2) was 9,623 compared with mean densities of 6,178 at Burlington Pier, Lake Ontario (Fitzsimons 1995b) and 4,250 at Stony Island, Lake Ontario (Perkins and Krueger 1995). Mean egg density (eggs/m2) among all sites using egg bags was 2,273 (mean = 436, excluding the Grand Isle breakwall) in Lake Champlain, 30.1 in Lake Superior (Kelso et al. 1995), 1,150 in Lake Ontario (Fitzsimons 1995b), 221.7 in Parry Sound, Lake Huron (J. Fitzsimons, Canada Centre for Inland Waters, unpublished data), and 17.8 in Lake Michigan (J. Jonas and R. Claramunt, Michigan Department of Natural Resources, unpublished data).
Artificial reefs comprised over half of the spawning sites we located; eggs were collected at five of eight artificial sites and three of six natural sites. Ore Den Bay and Iron Bay are adjacent to abandoned iron mining sites where large quantities of mine tailings have trickled down steep shorelines into the bays. Both sites have steep slopes and deep interstitial spaces. Hatching may be compromised at these sites as dense macrophyte beds develop during the summer and decompose over the winter, contributing organic matter to the substrate. Low egg densities at both sites and the failure to collect fry at Ore Den Bay suggest that these sites are relatively unproductive compared with other spawning sites within the lake. The Arnold Bay site is a small cobble crib covering a water intake line used as a lake trout stocking site from 1989 to 1994. It is unknown whether lake trout show spawning site fidelity; however, considerable evidence suggests that, in general, fish recognize and return to specific sites when displaced (Dodson 1988; Gunn 1995). Adequate egg incubating substrate and possible imprinting to the stocking site may explain the high egg and fry densities at Arnold Bay. The Saxton Cove site is located over a small, ruined breakwall with deep and extremely clean interstitial spaces. Although the rate of egg deposition was moderate compared with that of other sites within the lake, no fry were collected in spring; however, fry were subsequently collected at this site in 2003. The highest egg deposition rates of all sites occurred at the Grand Isle breakwall. The breakwall protects a ferry dock and is situated parallel to the ferry path and perpendicular to the prevailing winds. Both the ferry and the winds generate water currents around the breakwall, keeping the substrate clean of sand, silt, and fine organic matter. Water flowing through the Ed Weed Fish Culture Station empties into Lake Champlain, approximately 200 m from the breakwall. Chemical cues from several salmonid species reared at the hatchery (including lake trout) may aid in attracting spawning lake trout to the area. Egg deposition levels at this site may have had a negative effect on overall survival as fungus can rapidly spread if egg densities are too high. Several egg bags collected in the spring from the Grand Isle breakwall site had large fungus patches attached to the mesh resulting from decomposing eggs. While this is an artifact of the artificially high egg clustering within egg bags, it is still indicative of potential problems in the natural substrate.
Artificial sites without lake trout egg deposition include the two Burlington breakwall sites and Willsboro Bay. During the summer of 2001, the northern and southern ends of the Burlington breakwall were repaired by the U.S. Army Corps of Engineers. Angular cobble was piled at the base of the breakwall, creating deep, clean interstitial spaces. Prior to the repairs, no lake trout egg incubating substrate was present, so lake trout likely did not use those areas for spawning. Recent renovations to the two Burlington breakwall sites may explain why eggs were not collected from either site. The site in Willsboro Bay is a steep, straight shoreline where angular rock from railroad tracks has trickled into the water. Although this substrate is clean of organic matter and has deep interstices, spawning lake trout may have been attracted to other sites within the bay that we did not sample. Spawning behavior has been observed by lake biologists at Willsboro Point, which is in close proximity to the site we sampled.
Whallon Bay, Shelburne Point, and Button Bay are natural sites where eggs were collected. Although Arnold Bay and the Grand Isle breakwall had the highest densities of eggs and fry, Whallon Bay may be the most productive site based on reef area. Arnold Bay and the Grand Isle breakwall are small sites, approximately 189 and 570 m2 in area, respectively. The area of appropriate spawning substrate at Whallon Bay is over a hundred times larger, at approximately 64,372 m2. Egg bags were buried in a line at depths of 3–4 m. We did not observe any differences in habitat characteristics, such as substrate size or reef slope along the line of bags. However, since egg distribution among the egg bags was not uniform, it is possible that lake trout identified substrate characteristics that we could not. There was an obvious peak of egg deposition in the bags buried in the center of the line. We also observed eggs at depths of less than 2 m down to 9 m, suggesting that deposition is extensive at the Whallon Bay site. Low numbers of eggs were collected from Button Bay and Shelburne Point. Extensive habitat surveys have not been conducted at either site. Surface observations at these sites suggest that appropriate lake trout spawning substrate appears to be limited, as bedrock is the dominant substrate type.
Several seemingly adequate natural sites where eggs were sampled for, but not collected, included Allen Hill, Cannon Point, and Thompson's Point. Allen Hill and Cannon Point both have relatively shallow interstitial spaces that were “cleaner” during the summer, when habitat evaluations were conducted, than in the fall spawning season. Thompson's Point was chosen for egg sampling because in fall of 2001, scuba diver observations revealed over 20 adult lake trout at the site. These fish may have been staging to spawn somewhere else since the substrate here (bedrock covered with zebra mussels) is of low egg incubating quality and no eggs were collected.
Fry trapping efforts in 2001 and 2002 provided evidence that eggs were successfully incubating and hatching. Lake Champlain mean fry CPUE ranged from 0.08 to 2.38, compared with mean estimates of 0.35 in Lake Michigan, 0.04 in Lake Ontario, 0.02 in Lake Huron, and 0.57 in Lake Superior (Marsden et al. 1988). Considering the high levels of egg deposition at Grand Isle, fry CPUE was lower than expected; however, our traps may not have always been effectively fishing. The cobble substrate used to construct the breakwall does not extend very far from the base of the wall and is adjacent to sand. Some traps may not have been set on the cobble or may have slid off onto the sand; this would artificially lower CPUE. Also, predation by slimy sculpin may have lowered fry catch rates. For the first 2 weeks of fry trapping in spring 2002, up to 12 slimy sculpins were captured per trap and were likely feeding on lake trout fry in the traps. No fry were found in stomach contents, but this is not unexpected as digestion would have occurred before the traps were checked. We modified the fry traps to exclude predators and saw a decrease from 46 to 5 total sculpins collected from all traps and an increase in fry· trap−1·d−1 from 0.47 to 0.71.
Our data indicate that lake trout spawn at multiple sites throughout Lake Champlain and that mean egg density was higher than mean density at an already-restored population in the Great Lakes (Parry Sound, Lake Huron). In 2001 and 2002, mean fry CPUE in Lake Champlain exceeded maximum estimates from Lake Superior where lake trout are self-sustaining. It is important to note that although egg density and fry CPUE in Lake Champlain are high compared with those of the Great Lakes, total egg and fry production among these systems cannot be compared because the geographic extent of spawning is unknown. Spawning sites and egg deposition were easy to locate in Lake Champlain, and have been difficult to find in portions of the Great Lakes (e.g., Horns et al. 1989; Edsall et al. 1995). This difference is likely a consequence of scale; there are few if any extensive (>1 km) areas of cobble in Lake Champlain and few are far (>1 km) from shore, so spawning appears to be concentrated in small, readily accessible areas. Given the high numbers of eggs and fry we detected, we would expect to collect unclipped juveniles and adults in assessment surveys. There has been a very limited amount of sampling for unclipped juvenile lake trout in Lake Champlain; state assessments for lake trout focus on adult sampling. In limited sampling efforts, we did not collect any unclipped juvenile lake trout. The decrease in the percentage of unclipped adult lake trout during the early years (1980s) of adult sampling may be explained by an improvement in fin clipping and clip identification practices. If the entire fin is not clipped, regeneration occurs and the original clipping can be difficult to identify. Data beginning in the early 1990s are likely a more accurate representation of unclipped and presumably lake-produced fish as hatchery and sampling crews gained experience. Since 1990, a subsample of 100 lake trout have been checked for clips each year prior to stocking; 97–99% of these fish are clipped (B. Chipman, VTDFW, personal communication). Schneider et al. (1990) and Holey et al. (1995) suggested that successful natural recruitment beyond the fry stage can be construed when the number of unclipped adult lake trout in the population exceeds 2%, although the examination of an extensive tagging and clipping dataset in Lake Michigan suggests that 5% should be used as a baseline (J. Jonas, Michigan Department of Natural Resources, personal communication). The recent increase in the percentage of unclipped lake trout from just above 2% in 1993 to over 10% in 2000 suggests that lake trout spawned in the lake are recruiting to age-3 and older. However, from 1994 to the present, the length frequency of unclipped adult lake trout has also increased. In 1994, the most numerous size-class of unclipped lake trout was 350–450 mm, and each year since then the majority has come from a slightly larger length-class. In 2001, the 650–750 mm length-class contained the majority (75%) of unclipped lake trout. This suggests that in the late 1980s or early 1990s, one naturally produced year-class may have had particularly high survival or fish were mistakenly stocked without being clipped. The decrease in unclipped lake trout from 2000 (10.4%) to 2001 (5.7%) could potentially be a result of mortality of this larger and older year-class; very few lake trout caught in the assessment surveys are larger than 750 mm. Even if the unclipped lake trout were mostly natural recruits, the percentage of unclipped lake trout is lower than expected considering the high levels of egg and fry production.
A decrease in stocking rate could potentially overinflate the percentage of unclipped lake trout in the population. Because the annual stocking rate in Lake Champlain was variable and decreased by half since 1995, we examined whether this variation affected the ratio of unclipped to clipped fish. The percentage of unclipped lake trout was adjusted by the number of yearling equivalents stocked (i.e., each age-class of clipped lake trout recovered in a given year was adjusted by the total number of yearlings stocked that made up that year-class). This adjustment produced a trend identical to the nonadjusted percentage of unclipped lake trout, indicating that variation in stocking rate did not affect the percentage of unclipped fish.
Many of the factors impeding lake trout recruitment in the Great Lakes are lacking in Lake Champlain (e.g., insufficient spawning stock, contaminants, lack of genetic diversity, EMS, and predation on age-0 lake trout by exotic species). The high levels of egg and fry abundance in Lake Champlain, the failure to collect lake-produced juveniles, and the pattern of increasing length in the percentage of unclipped fish all suggest a bottleneck during the postemergent fry life stage. Little is known about habitat for, or predators of, this life stage. Potential lake trout fry predators in the lake include slimy sculpins, yellow perch Perca flavescens, and rainbow smelt (Jones et al. 1995). Yellow perch are very abundant and have been observed at several spawning sites during fall and spring. Further studies that examine egg and fry production and survival are necessary to better understand impediments to lake trout restoration in Lake Champlain. In particular, studies of the abundance and diets of potential predators of eggs, sac fry, and postemergent fry may explain why the high levels of egg abundance we document have not resulted in the recruitment of naturally produced lake trout.
Acknowledgments
We thank John Fitzsimons, Jory Jonas, and Randy Claramunt for field assistance and for making comments on this manuscript. We also thank Jesse Wheeler, Mark Beekey, Matt Toomey, Chris Lapointe, Dan Laven, Aaron Pepperman-Taylor, Eric Carlson, Captain Richard Furbush of the RV Melosira, and Captain Bob Gay of the RV Shark for assistance with field sampling and data collection. We thank Kevin Kelsey and Dan Marchant of the Ed Weed Fish Culture Station for their assistance. Funding was provided by the Great Lakes Fishery Commission, the Great Lakes Fishery Trust, and the National Undersea Research Program. We also greatly appreciate financial contributions provided by the Vermont Department of Fish and Wildlife and F. Peter Rose.




