Coupling of Protonation Switches During Rhodopsin Activation†
This paper is part of the Proceedings of the 12th International Conference on Retinal Proteins held at Awaji Island, Hyogo, Japan on 4–8 June 2006.
Abstract
Recent studies of the activation mechanism of rhodopsin involving Fourier-transform infrared spectroscopy and a combination of chromophore modifications and site-directed mutagenesis reveal an allosteric coupling between two protonation switches. In particular, the ring and the 9-methyl group of the all-trans retinal chromophore serve to couple two proton-dependent activation steps: proton uptake by a cytoplasmic network between transmembrane (TM) helices 3 and 6 around the conserved ERY (Glu-Arg-Tyr) motif and disruption of a salt bridge between the retinal protonated Schiff base (PSB) and a protein counterion in the TM core of the receptor. Retinal analogs lacking the ring or 9-methyl group are only partial agonists—the conformational equilibrium between inactive Meta I and active Meta II photoproduct states is shifted to Meta I. An artificial pigment was engineered, in which the ring of retinal was removed and the PSB salt bridge was weakened by fluorination of C14 of the retinal polyene. These modifications abolished allosteric coupling of the proton switches and resulted in a stabilized Meta I state with a deprotonated Schiff base (Meta ISB). This state had a partial Meta II-like conformation due to disruption of the PSB salt bridge, but still lacked the cytoplasmic proton uptake reaction characteristic of the final transition to Meta II. As activation of native rhodopsin is known to involve deprotonation of the retinal Schiff base prior to formation of Meta II, this Meta ISB state may serve as a model for the structural characterization of a key transient species in the activation pathway of a prototypical G protein-coupled receptor.
Introduction
Rhodopsin is the visual pigment responsible for dim-light vision mediated by vertebrate rod photoreceptor cells. Activation of rhodopsin is triggered by light isomerization of the 11-cis retinal protonated Schiff base (PSB) to all-trans, switching this covalently bound ligand from an inverse agonist to an agonist conformation. These initial changes within the retinal-binding pocket in the transmembrane (TM) domain of the receptor propagate into the receptor protein leading eventually to a photoproduct equilibrium between the inactive Meta I receptor conformation and the active Meta II state (1–3). Meta II binds and activates the visual heterotrimeric G protein transducin and triggers thereby the visual signal transduction cascade. Rhodopsin belongs to the super family of G protein-coupled receptors and serves as a prototype for biophysical studies on this physiologically important class of seven TM helical receptors.
The X-ray structures obtained from the inactive dark state (4–6) have revealed the existence of interhelical receptor microdomains (1) that undergo conformational changes during activation of rhodopsin (Fig. 1). These microdomains comprise interhelical networks between the TM helices H1, H2 and H7 and between H3 and H5, which contain the carboxylic acids Asp83 (H2) and Glu122 (H3), respectively. Both of these residues experience changes of their hydrogen-bonding properties during activation. These changes give rise to characteristic signatures in Fourier-transform infrared (FTIR) difference spectra, such that these carboxylic acids serve as sensitive and site-specific reporter groups for conformational changes in their respective microdomains (7,8).
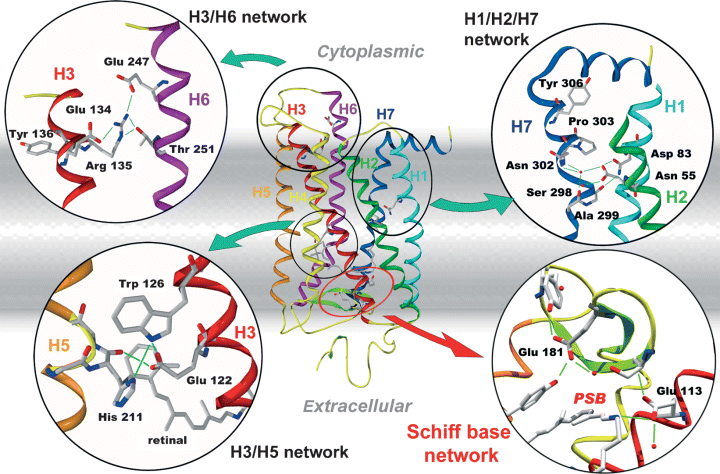
Molecular model of rhodopsin based on the structure of the inactive dark state. The inserts show close-ups of functional receptor microdomains that undergo changes during activation. The insert showing the Schiff base network is oriented with the cytoplasmic side facing down. The model is based on the co-ordinates PDB 1GZM6.
Two other microdomains are known to undergo proton-dependent reactions during the transition from the inactive Meta I to the active Meta II photoproduct state: the Schiff base network contains a salt bridge between the retinal protonated Schiff base (PSB) and a protein counterion, Glu113 (9,10), which maintains the inactive conformation of the dark state and of the earlier photointermediates. The salt bridge is broken in the transition from Meta I to Meta II by a net proton transfer from the PSB to Glu113 (11). In addition, the cytoplasmic H3/H6 network, including Glu134, is known to be involved in a proton uptake reaction abolished in the E134Q mutant (12), which appears to be responsible for the pH dependence of the Meta I/Meta II conformational equilibrium (2, 3).
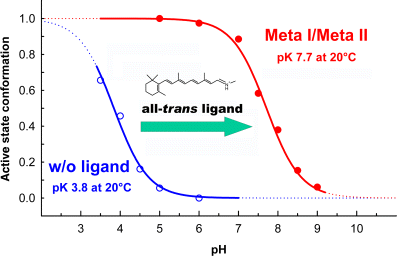
Titration curves of the pH-dependent equilibria between the active Meta II and inactive Meta I photoproduct conformations of rhodopsin (closed circles) and between active and inactive conformations of the apoprotein opsin (open circles) in their native membrane environment. The titration curves are based on conformationally sensitive band patterns of Fourier-transform infrared difference spectra. Presence of the agonist all-trans retinylidene protonated Schiff base ligand in the retinal-binding pocket in the Meta states shifts the apparent pKA of the equilibrium by about 4 units compared with the opsin equilibrium.
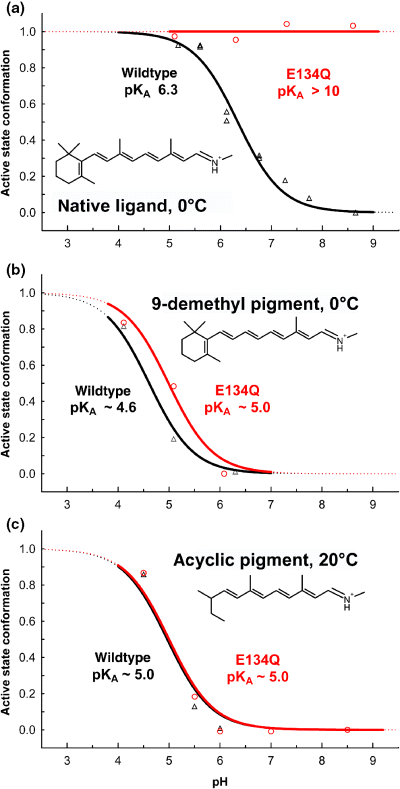
The E134Q mutation leads to pH-independent formation of the active state photoproduct Meta II in pigment with the native retinal chromophore (a), but not with the partial agonist retinal analogs 9-demethyl retinal (b) and acyclic retinal (c). The graph shows titration curves of the pH-dependent photoproduct equilibrium between active Meta II and inactive Meta I conformations of wild type (triangles) and E134Q mutant (circles) pigments reconstituted into phosphatidyl choline lipid membranes and regenerated with the respective retinals. The titration curves of the wild type 9-demethyl and acyclic pigments have considerably lower apparent pKA values as compared with the wild type pigment with the native retinal chromophore, reflecting their partial agonist nature. The corresponding shift of their photoproduct equilibrium to the inactive Meta I side is not relieved by the E134Q mutation. The ligands are shown in this graph as the all-trans isomers of the retinylidene protonated Schiff bases. Regeneration of the dark states of the pigments was achieved with the respective 9-cis retinal isomers.
In the absence of the retinal ligand, the apoprotein opsin forms a pH-dependent equilibrium between active and inactive conformations (13), that is shifted considerably to the inactive side, such that opsin adopts its active conformation only at very acidic pH (Fig. 2). Presence of the all-trans retinylidene Schiff base in the binding pocket of the Meta I/Meta II photoproduct increases the apparent pKA of the equilibrium by about 4 units as compared with the opsin equilibrium in the absence of ligands, corresponding to a shift of the ratio between active and inactive conformations, R*/R, by a factor of 104. The all-trans retinylidene Schiff base is therefore a full agonist. As the position of the Meta I/Meta II equilibrium depends exquisitely on the environment of the receptor and is essentially abolished in most commonly used detergents, it is very important to maintain largely native conditions. Therefore, we carried out experiments to determine the precise structural requirements for the agonist properties of the all-trans ligand mostly in native disk membranes or, in some cases, after reconstitution of purified mutant pigments into lipid membranes.
The 9-methyl group and the ring of retinal are important for efficient receptor activation
The retinal ligand of rhodopsin can be readily exchanged for modified synthetic ligands by bleaching rhodopsin in the presence of hydroxylamine, washing and adding a synthetic ligand, which leads to more or less efficient spontaneous regeneration of the corresponding artificial pigment. Such studies have revealed many details about chromophore–protein interactions (14,15). In the following, we will focus on two modifications that have been revealed in previous studies to interfere substantially with receptor activation, namely removal of the 9-methyl group (e.g. References [16–21]) and deletion of the ring of retinal (e.g. References [22–24]). The resulting artificial pigments, 9-demethyl and acyclic rhodopsin (or isorhodopsin, if the regeneration employed the 9-cis retinal isomer), form Meta I/Meta II photoproduct equilibria that are considerably shifted to the inactive Meta I product, corresponding to a lowering of the apparent pKA of the equilibrium (Fig. 3, black curves).
In native rhodopsin, the transition from Meta I to Meta II is driven by a positive entropy term TΔS, which is partially offset by a likewise positive enthalpy change ΔH (Table 1). In the 9-demethyl and the acyclic pigments, this positive entropy change of the transition is substantially decreased, which appears to reflect an increase of the entropy of their respective Meta I states (21,23). This increased entropy of Meta I is in turn due to the lack of important ligand–protein interactions that otherwise constrain the inactive Meta I conformation.
| ΔH (kJ/mol) | TΔS (kJ/mol at 20°C) | |
|---|---|---|
| Native* | 84 | 127 |
| 9-demethyl† | 46 (−38) | 71 (−56) |
| acyclic* | 69 (−15) | 97 (−30) |
| 14-F‡ | 46 (−38) | 92 (−35) |
| 14-F acyclic‡,§ | −23 (−107) | 5 (−122) |
An allosteric coupling between protonation switches is mediated by the 9-methyl group and the ring of retinal
Glu134 in the cytoplasmic H3/H6 network (Fig. 1) is part of the E(D)RY motif, which is conserved in class A members of the G protein-coupled receptor super family. Neutralization of Glu134 in the E134Q mutant significantly alters the Meta I/Meta II equilibrium and leads to exclusive formation of Meta II over the entire accessible pH range (Fig. 3a, circles). Replacement of Glu134 by a glutamine mimics therefore the cytoplasmic proton uptake reaction that is controlled by the H3/H6 microdomain (12). In particular, the mutationally induced Meta II photoproduct state of the E134Q mutant is associated with the regular net proton transfer from the PSB to Glu113, as evident from the corresponding FTIR-difference spectra. The two protonation switches, residing in the cytoplasmic H3/H6 network and in the PSB salt bridge, appear therefore to be allosterically coupled, such that cytoplasmic proton uptake induces the net proton transfer from the PSB to Glu113, disrupting the PSB salt bridge (25).
Surprisingly, this very pronounced effect of the E134Q mutation in pigment with native retinal is largely absent in the artificial pigments reconstituted with 9-demethyl or acyclic retinals (Fig. 3b,c, circles) (21,23). In the E134Q mutants of 9-demethyl and acyclic pigments, the pH dependence of Meta I/Meta II is maintained and its apparent pKA is hardly affected by the mutation, in strong contrast to pigment containing native retinal. In the 9-demethyl and acyclic pigments, the allosteric coupling between the H3/H6 microdomain and the PSB salt bridge is therefore obviously broken. The PSB salt bridge is not neutralized and inactive Meta I conformations are maintained in the photoproduct of these pigments even if the cytoplasmic proton uptake reaction is mimicked by the E134Q mutation. Only at very low pH, their Meta I products are converted to active Meta II, possibly by protonation of Glu113 from the solvent.
The 9-methyl group and the ring of retinal are therefore necessary for co-ordinating the helix movements responsible for the allosteric coupling between the H3/H6 and the PSB microdomains. A possible mechanism for this coupling and for its breakdown in the absence of ring and 9-methyl group of all-trans retinal is suggested by recent NMR studies, showing that all-trans retinal moves along its polyene axis during activation, clashing with its ring into the H3/H5 network around Glu122 (26,27). This translational motion is likely directed by the 9-methyl group of retinal, which appears to move in a channel formed by protein residues lining the binding pocket.
The allosteric coupling operates in both directions
Does this allosteric coupling work in both directions, such that weakening of the PSB salt bridge would increase the apparent pKA of the cytoplasmic proton uptake reaction? This could, in principle, be tested by disrupting the PSB salt bridge (e.g. by neutralization of the Glu113 counterion in an E113Q mutant). This is, however, not a particularly useful approach, as it considerably perturbs the structure of the dark state (9,10). Therefore, we lowered the stability of the PSB salt bridge by introducing an electron withdrawing fluorine group at C14 of the polyene (Fig. 4a). The effect of lowering the intrinsic pKA of the PSB is the same as from removing the PSB counterion but the degree of structural perturbation is slight. As the pKA of the PSB is extremely high in the dark state (>16) (28), the fluorination-induced drop of its intrinsic pKA by approximately 2 units is expected not to alter the dark state excessively. In the Meta I photoproduct state, however, the pKA of the PSB can be expected to be substantially lower, such that fluorination of C14 should alter the properties of the Meta I/Meta II equilibrium significantly.
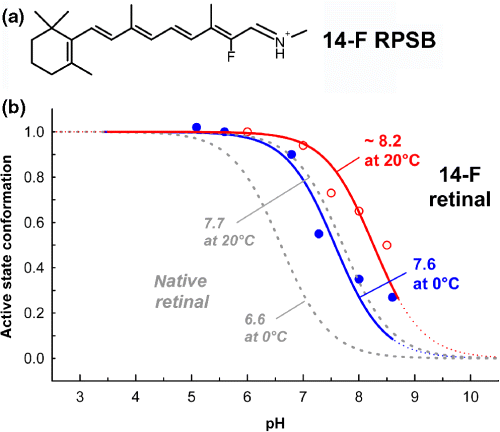
Weakening of the PSB salt bridge by fluorination of C14 of the polyene in the 14-F retinylidene protonated Schiff base (RPSB) (a) increases the apparent pKA of the Meta I/Meta II equilibrium in the 14-F pigment (as compared with native pigment shown in gray) due to the allosteric coupling between the PSB and the cytoplasmic H3/H6 microdomains (b). This weakening of the salt bridge lowers the positive enthalpy change of the transition from Meta I to Meta II by approximately 45%, as revealed by a thermodynamic analysis of these titration curves (see Table 1).
This is indeed the case, as shown in Fig. 4b. Weakening of the PSB salt bridge by retinal fluorination increases the pKA of the cytoplasmic proton uptake reaction and shifts therefore the Meta I/Meta II equilibrium toward Meta II (29). The allosteric coupling between the H3/H6 and the PSB microdomains is bidirectional, such that alteration of one of the two protonation switches affects the other in reciprocal fashion. A thermodynamic analysis of the titration curves of the 14-F Meta I/Meta II equilibrium reveals that the associated enthalpy change of the transition from Meta I to Meta II is reduced by the weakening of the PSB salt bridge to approximately half of that observed in the native pigment (Table 1).
Stabilizing a Meta I state with deprotonated schiff base
As we have seen above, in the Meta I/Meta II equilibrium of rhodopsin with native retinal the PSB salt bridge is strong and also the allosteric coupling between PSB and H3/H6 microdomains is strong. In the 14-F pigment, the salt bridge is considerably weakened, but the coupling between the microdomains is maintained, while in the acyclic pigment, the salt bridge remains strong and the coupling is abolished. By combining polyene fluorination with deletion of the ring of retinal in a 14-F acyclic pigment (Fig. 5a), we would expect a Meta I/Meta II equilibrium in which both the PSB salt bridge and the allosteric coupling to the cytoplasmic proton uptake group are weakened.
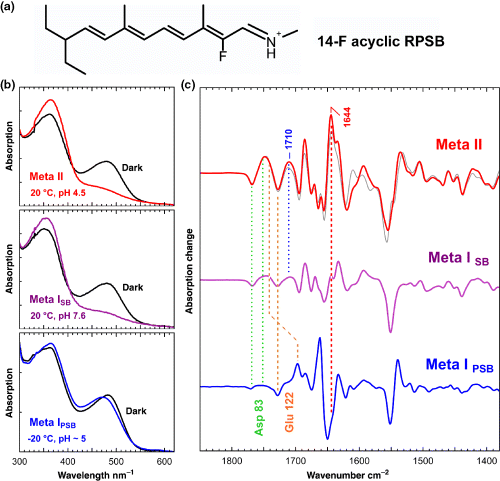
The 14-F acyclic pigment (shown as the RPSB in a) forms a Meta I state with deprotonated Schiff base (Meta ISB), as evident from its 370 nm absorption peak (b, middle panel), which is distinctly different from that of Meta I with protonated Schiff base (Meta IPSB) formed at low temperature (b, lower panel). A structural characterization of this unusual Meta ISB state (c, middle), as compared with Meta II (c, top) and with Meta IPSB (c, bottom), can be achieved using the corresponding Fourier-transform infrared photoproduct minus dark state difference spectra. In Meta ISB, the conformations of the microdomains formed by the H1/H2/H7 network, the H3/H5 network and the Schiff base network are already partially Meta II-like as indicated by the absorption changes of Asp83, Glu122 and of the at least partially protonated counterion at 1710 cm−1, respectively. The amide I marker band of Meta II at 1644 cm−1, on the other hand, is still Meta I-like in Meta ISB and appears only after the cytoplasmic proton uptake reaction and the transition to full Meta II of this synthetic pigment, which appears similar as native Meta II (c, top, gray spectrum).
The pigment with the 14-F acyclic ligand indeed shows a surprising behavior (29): at −20°C, where the conformational transition to Meta II is completely blocked, a Meta I photoproduct state with a protonated Schiff base (Meta IpSB) is formed with conformational features that are similar to those of Meta I of nonfluorinated acyclic pigment (Fig. 5b,c, lower panels). At temperatures above −20°C, where the conformational equilibrium with Meta II is established, the Schiff base in Meta I is deprotonated (Meta ISB), such that the resulting Meta ISB state becomes indistinguishable from Meta II in UV–visible spectra (Fig. 5b). Only in the FTIR difference spectra Meta ISB can be distinguished from the isospectral Meta II state (Fig. 5c). A structural analysis of the FTIR-difference spectra reveals that the conformation of Meta ISB is distinctly different from that of Meta IPSB formed at low temperature. In Meta ISB, several of the receptor microdomains are in conformations that are already partially Meta II-like, as the H1/H2/H7 microdomain with Asp83 as reporter group and the H3/H5 microdomain with Glu122 as reporter group. Also the Schiff base domain is altered. Besides the deprotonation of the Schiff base, we observe an at least partial protonation of the protein counterion, as evident from the positive photoproduct band at 1710 cm−1, which can be assigned to a carboxylic acid due to its H/D sensitivity and which is not associated with Glu122, as confirmed using a corresponding site-directed mutant (29). The conformational equilibrium between Meta ISB and Meta II is still pH dependent, indicating that the cytoplasmic proton uptake reaction takes place only in the transition to Meta II. This was confirmed using the E134Q mutant of 14-F acyclic, which extended formation of Meta II into the alkaline range. The cytoplasmic proton uptake reaction appears therefore to be associated with the formation of the amide I marker band of Meta II at 1644 cm−1, indicating possibly local structural changes of the protein backbone due to movement of TM H6 during the transition to Meta II.
Dissecting the transition from Meta I to Meta II into two partial reactions
The stabilization of Meta ISB in the 14-F acyclic pigment and the resulting unique possibility of a structural characterization of this unusual state is particularly appealing. During activation of native pigment, a Meta I species with deprotonated Schiff base is formed transiently prior to formation of Meta II, as has been shown by time-resolved UV–visible spectroscopy of the physiological photointermediates in a native membrane environment (30) where a Meta I380 state forms transiently accounting for one of two branches leading to Meta II. This agrees also with time-resolved proton uptake experiments on detergent solubilized rhodopsin, where a probably related species was termed Meta IIA (31).
Based on these observations, we can dissect this transition from Meta I to Meta II into two partial reactions (Fig. 6): (1) a disruption of the PSB salt bridge, which leads to a Meta ISB state and the associated conformational changes described above and (2) the cytoplasmic proton uptake reaction, which leads to the final transition to the full Meta II conformation.
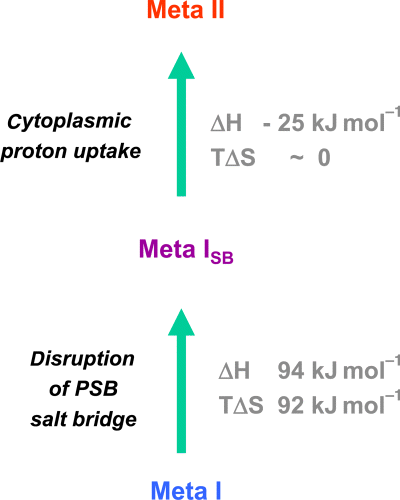
The transition from Meta I to Meta II in the acyclic pigment analogs involves two partial reactions, disruption of the protonated Schiff base (PSB) salt bridge and subsequent cytoplasmic proton uptake. The first partial reaction leads to Meta I with deprotonated Schiff base, which is transiently formed also during activation of native rhodopsin and which can be studied as a stable species in the 14-F acyclic synthetic pigment. The data obtained with the acyclic analogs indicate that the first partial reaction, disruption of the PSB salt bridge, is energetically by far the more relevant step as compared with the subsequent proton uptake reaction.
Using the thermodynamic data obtained for the acyclic pigments (Table 1), approximate values of ΔH and TΔS can be derived for both partial reactions (Fig. 6). These indicate that disruption of the PSB salt bridge is energetically by far the more relevant step as compared with the cytoplasmic proton uptake reaction. It is associated by large positive values of both the enthalpy change ΔH and the entropy term TΔS, while the proton uptake reaction is enthalpically slightly downhill at approximately constant entropy. Although these values for the partial reactions were derived from the acyclic analogs, they apply as qualitative estimates also for the corresponding partial reactions in the native pigment.
Conclusions
By combining modifications of the retinal ligand with site-directed mutagenesis of the receptor protein, an allosteric coupling mechanism between the two protonation switches, disruption of the PSB salt bridge and cytoplasmic proton uptake, was established. Using site-directed mutagenesis and chromophore fluorination, both protonation switches can be modulated independently. A breakdown of this coupling in the presence of 9-demethyl and acyclic all-trans retinal ligands appears to be the reason for their partial agonist properties. By using a synthetic 14-F acyclic analog, in which the PSB salt bridge and this allosteric coupling were weakened simultaneously, a Meta I state with deprotonated Schiff base could be stabilized. This state could serve as a structural template for corresponding species formed transiently during activation of the native pigment.
Acknowledgments
Acknowledgements— This work was supported by grants from the D.F.G. (to F.S. and R.V.), Fonds der Chemischen Industrie (to F.S.) and the Israel National Science Foundation (to M.S.). T.P.S. is a senior scholar of The Ellison Medical Foundation. M.S. holds the Katzir-Makineni professorial chair in chemistry.




