Low Cell Motility Induced by hsp27 Overexpression Decreases Osteolytic Bone Metastases of Human Breast Cancer Cells In Vivo
Abstract
The mechanisms controlling the formation of osteolytic bone metastases in patients with breast cancer are still poorly understood. To explore the role of motility in the establishment of osteolytic bone metastases, we have used a model of bone metastasis in which MDA-MB-231 breast cancer cells exhibiting low (hsp27-transfectants) and high (control-transfectant) endogenous cell motility were compared. We found that MDA-MB-231 cells exhibiting low cell motility were less capable of establishing osteolytic lesions. The number and the area of the osteolytic lesions in mice inoculated with low motility cells were both significantly smaller. Histomorphometry of bone lesions also demonstrated less tumor area in mice bearing hsp27 transfectants although there was no difference in the osteoclast number per square millimeter of tumor–bone interface. These data suggest that cell motility may be an important mechanism in the metastatic cascade of breast cancer cells to the bone and that controlling cell motility may be a useful target to prevent the establishment of osteolytic bone metastases.
INTRODUCTION
OSTEOLYTIC BONE METASTASES are a frequent clinical problem in patients with breast cancer.1 Despite their clinical importance and consequences, such as pain, pathological fracture, hypercalcemia, and nerve compression, the pathophysiological process of osteolytic bone metastases formation is still incompletely understood. There is overwhelming support that the expression of matrix metalloproteinases by tumor cells is necessary to detach from the primary tumor site in order to reach the circulation.2-4 However, once breast cancer cells are in circulation and still accessible for treatment, little is known about the cellular activities influencing the formation and establishment of osteolytic bone metastases.
Osteolytic lesions are the result of a paracrine synergy that takes place between osteoclasts and breast cancer cells present in the bone environment.5, 6 Breast cancer–derived products, such as parathyroid hormone-related protein (PTHrP), mediate bone destruction by stimulating osteoclastic bone resorption.7 This stimulated bone resorption then contributes to the release of more bone-derived growth factors present in the mineralized bone matrix.8 Many of these bone-derived growth factors have been shown to be chemotactic and therefore may also contribute to the chemotaxis of breast cancer cells in the bone environment.9, 10 Thus, the capacity to respond to bone-derived chemoattractants may be a prerequisite for breast cancer cells to establish themselves into the bone matrix.
To explore the role of motility in the establishment of osteolytic bone metastases, we used a bone metastasis model in which stably transfected MDA-MB-231 breast cancer cells exhibiting low endogenous motility in vitro due to hsp27 overexpression11 were compared for their capacity to form osteolytic bone metastases. In this previous study,11 we showed that hsp27 overexpression resulted in increased in vitro and in vivo invasiveness and cellular proliferation but decreased endogenous cell motility of breast cancer cells. However, both the low transfected and the highly motile untransfected MDA-MB-231 cells were capable of exhibiting a chemotactic response to serum. In this present study, the effect of hsp27 overexpression and consequent decreased endogenous cell motility was investigated in a mouse model of human breast cancer metastasis to the bone.
MATERIALS AND METHODS
Transfection of MDA-MB-231 cells
MDA-MB-231 human breast cancer cells, growing exponentially in monolayer cultures, were maintained in modified Eagle's medium supplemented with 10% fetal bovine serum, 6 ng/ml insulin, and 25 μg/ml gentamicin sulfate. Cells were transfected as previously described.12 A full-length human hsp27 cDNA was prepared by polymerase chain reaction amplification and subcloned into the EcoRI site of the mammalian expression vector pcDNAI (Invitrogen, San Diego, CA, U.S.A.), where expression is under the control of a constitutive CMV promoter. Hsp27-transfectants were screened first for genomic integration by polymerase chain reaction and second by Western blot analysis using an anti-human hsp27 monoclonal antibody (1:100) (clone G3.1; Neomarkers, Fremont, CA, U.S.A.), followed by incubation with an HRP-conjugated anti-mouse immunoglobulin G antibody detected by ECL (Amersham Life Sciences, Buckinghamshire, U.K.). Colonies of MDA-MB-231 cells were picked with cloning cylinders approximately 3 weeks after selection in media containing 600 μg/ml of G418. Transfectants were then expanded and maintained on G418 for at least 1 month after picking.
Experimental protocols
Intracardiac injection of MDA-MB-231 cells was performed according to the technique described previously.7, 13 Subconfluent MDA-MB-231 cells were fed with fresh medium 24 h before intracardiac injections. Cells (105) were trypsinized, washed, and resuspended in 0.1 ml of phosphate-buffered saline. Cell suspensions were inoculated using a 27-gauge needle into the left heart ventricle of 4–6 week-old female BALB/c nude mice (Audie Murphy Veteran's Administration Hospital, San Antonio, TX, U.S.A.). Water and autoclaved mouse chow (Ralston Purina Co., St. Louis, MO, U.S.A.) were provided ad libitum. Blood for whole blood ionized calcium concentrations was drawn by retro-orbital puncture from mice under metofane anesthesia once per week. Body weight was measured at that time as well. Three in vivo experiments were performed. In the first two experiments, there were five animals per group, and ten animals per group in the third experiment. In each experiment, mice were the same age (4–6 weeks) and had similar body weights at the time of tumor inoculation. The duration of each experiment was 31 days from the time of tumor inoculation until sacrifice.
Ca2+ measurements
Ca2+ concentrations were measured using a Ciba Corning 634 ISE Ca++/pH analyzer (Corning Medical and Scientific, Medfield, MA, U.S.A.) and adjusted to pH 7.4.14 Samples were run in duplicate and the mean value recorded.
PTHrP assay
To test the effect of the bone-derived growth factor transforming growth factor-β (TGF-β) on PTHrP secretion by MDA-MB-231 cells, 104 cells/ml were plated onto 48-well plates. When near confluence, cells were washed with phosphate-buffered saline, and 250 μl of serum-free Dulbecco's modified Eagle's medium containing TGF-β1 (5 ng/ml) was added. TGF-β1 was purchased from R&D Systems (Minneapolis, MN, U.S.A.). Conditioned media were collected after 48 h and stored at −70°C for PTHrP measurement. Cell number was counted for each well to correct the PTHrP concentration of the conditioned media. Triplicate measurements were performed. PTHrP concentrations were measured in conditioned media using a two-site immunoradiometric assay (Nichols Institute, San Juan Capistrano, CA, U.S.A.) that uses two polyclonal antibodies specific for the NH2-terminal-(1–40) and -(60–72) portions of PTHrP and has a sensitivity of 0.3 pM.15 PTHrP concentrations were calculated from a standard curve using Prism (GraphPAD Software for Science, San Diego, CA, U.S.A.) on an IBM compatible computer. PTHrP concentrations in conditioned media samples were calculated from a standard curve generated by adding recombinant PTHrP-(1–86) to the specific type of medium being tested and were considered undetectable if media concentrations were <0.3 pM before correction for cell number.
Radiographs and measurement of osteolytic lesion area
Whole-body X-rays were done on all animals. Animals were radiographed in a prone position against film (X-Omat AR; Eastman Kodak Co., Rochester, NY, U.S.A.) and exposed at 35 KVP for 6 s using a Cabinet X-ray System-Faxitron Series (43855 A; Faxitron Corp., Buffalo Grove, IL, U.S.A.). Films were developed using a Konica film processor (Konica, Wayne, NJ, U.S.A.). The area of osteolytic lesions was measured in both fore- and hindlimbs of all mice using an image analysis system in which radiographs were visualized using a fluorescent light box (Kaiser, Germany) and Macro TV Zoom lens 18–108 mm f2.5 (Olympus Corp., Tokyo, Japan) attached to a video camera (DXC-151; Sony Corp., Tokyo, Japan). Video images were captured using a frame grabber board (Targa+; Truevision, Armonk, NY, U.S.A.) with an IBM compatible 486/33 MHz computer. Quantitation of lesion area was performed using image analysis software (Jandel Video Analysis; Jandel Scientific, Corte Madera, CA, U.S.A.). Data are represented as total lesion area and number per animal. Quantitation was performed by a single investigator (M.D.) who was blinded to the experimental groups.
Bone histology and histomorphometry
Bone histomorphometry was performed on bones from the third experiment in which n = 10. Fore- and hindlimb long bones were removed from mice at time of killing, fixed in 10% buffered formalin, decalcified in 14% EDTA, and embedded in paraffin wax. Sections were cut using a standard microtome, placed on poly L-lysine–coated glass slides and stained with hematoxylin, eosin, orange G, and phloxine. Total tumor area and osteoclasts number expressed per millimeter of tumor/bone interface were measured in midsections of tibiae and femora without knowledge of treatment groups, to assess tumor involvement. Histomorphometric analysis was performed on an OsteoMeasure System (Osteometrics, Atlanta, GA, U.S.A.) using an IBM compatible computer. Four to six long bones were analyzed per animal. For each bone, four to six levels were cut and each level was assessed to determine the one most representative for histomorphometric analysis. Representative sections were defined as those which traversed the midportion of each bone and which also contained the maximum amount of tumor. Tumor area was quantitated at the ×4 objective, and osteoclast number per minute of tumor/bone interface was quantitated at ×10.
Statistical analysis
Analysis of variance was used to test for differences due to hsp27 expression and motility. The analysis included a “blocking” factor for experimental replication, in order to adjust for between-replication differences. Clonal variability, among clones of the same type (control vs. hsp27-transfected), was accounted for by including a nested factor (clone within type). Analyses were conducted using SAS version 6.09 (SAS Institute, Cary, NC, U.S.A.). All in vivo results are expressed as the mean ± SEM. Data were analyzed by repeated measures analysis of variance followed by Tukey–Kramer post-test. p values of <0.05 were considered significant.
RESULTS
Formation of osteolytic bone metastases
The MDA-MB-231 clonal cell lines used in this study were previously described elsewhere.11 MDA-MB-231 cell lines (clones 19 and 12(2)) stably overexpressing hsp27 demonstrated decreased endogenous cell motility compared with cells transfected with only the empty vector (control clone 1) or parental MDA-MB-231 cells expressing low endogenous levels of hsp27 in vitro.11 Because of their altered cell motility, clones 19 and 12(2) were used in this study to evaluate the role of motilty in the establishment of osteolytic bone metastases.
Figure 1 illustrates representative radiographs taken 30 days post–tumor inoculation of clones 19 and 12(2) and control clone 1. Osteolytic lesions were greater in mice inoculated with control clone 1, which exhibits high cell motility in vitro, compared with mice inoculated with clones 19 and 12(2). Bone destruction was quantitated from long bones by a computerized image analysis system. The number (Fig. 2A) and the area (Fig. 2B) of the lesions were both found to be significantly smaller in the mice inoculated with hsp27 overexpressing clones 19 and 12(2) (p < 0.001).

Radiographs of representative osteolytic bone lesions in hindlimbs of MDA-MB-231–bearing nude mice. (Left panel) Mice inoculated with high motility cells (control clone 1). (Middle panel) Mice inoculated with low motility cells (clone 19). (Right panel) Mice inoculated with low motility cells (clone 12(2)). Note the difference of the area of the osteolytic bone lesion in the left panel.
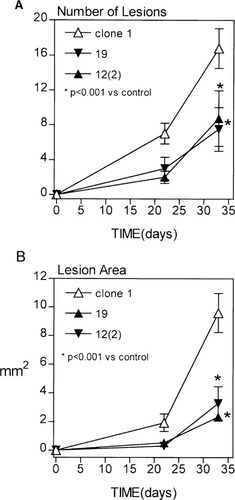
The number and area of osteolytic bone metastases in nude mice bearing high and low motility cells. (A) The number and (B) the area of osteolytic bone metastases were scored at day 21 and 30 after cell inoculation on radiographs using quantitative computerized image analysis. Each group had five mice. Values shown are mean ± SE. (*p < 0.001 vs. control clone 1). The experiments were performed three times and the same results obtained. The data shown here are from one experiment.
Visual and histomorphometry analyses
During the course of these experiments, mice inoculated with control clone 1 became paraplegic before the mice inoculated with either clones 19 and 12(2) (results not shown). This may reflect a more severe tumor burden in these control clone 1 mice. At the time of sacrifice, ∼70% of the mice inoculated with control clone 1 were paraplegic as opposed to 20% of mice inoculated with clones 19 and 12(2). Body weight declined significantly in all groups, but the decline was greatest in mice bearing the control clone 1 compared with those clones overexpressing hsp27 (Fig. 3A).

Visual and histomorphometric analyses from mice inoculated with high and low motility MDA-MB-231 cells. (A) Body weight and (B) (left panel) tumor area (mm2) and (right panel) Osteoclasts number per square millimeter of tumor adjacent to bone (tumor/bone interface). n = 10 per group. Values represent the mean ± SEM.
Histomorphometric analysis of long bones confirmed the radiographic findings. Tumor area in bone was greater in mice bearing the control clone 1 tumors compared with either of the hsp27 overexpressing clones (Fig. 3B). However, osteoclast number per square millimeter of tumor–bone interface did not significantly differ between the groups. Whole blood ionized calcium concentrations remained normal throughout the duration of the experiment (data not shown).
PTHrP secretion
To ensure that stable transfection of hsp27 did not alter the ability of clones 19 and 12(2) to secrete tumor products that promote bone destruction,7 we assessed the secretion of the most potent of these tumor-secreted products, PTHrP, in the transfectants, the parental cells, and two other control cell lines. PTHrP concentrations were measured in conditioned media in the basal state as well as in response to TGF-β since high concentrations of TGF in the bone microenvironment likely stimulate PTHrP production by tumor cells. There was no difference in basal or TGF-stimulated PTHrP production between the hsp27 overexpressing clones 19 and 12(2) and the control clone 1 (Fig. 4). These three breast cancer cell lines produced significantly less PTHrP than a human squamous cell carcinoma of the lung, RWGT2, derived from a patient with hypercalcemia and bone metastases,14 but significantly more than CHOK1 cells (Fig. 4). All secrete ∼2 pM of PTHrP normalized to 1 × 105 cells, suggesting that the reduction of osteolytic bone metastases formation seen in mice inoculated with clones 19 and 12(2) was not due to a loss of PTHrP secretion.
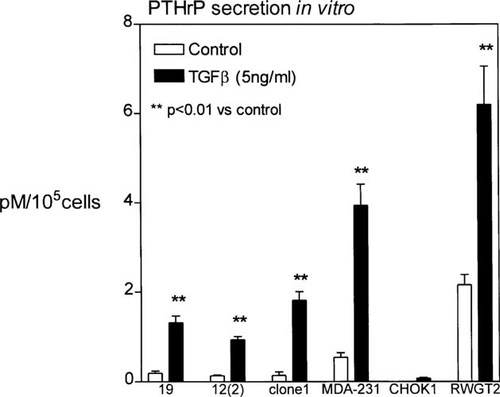
PTHrP secretion in response to TGF-β stimulation. Cells were stimulated with 5 ng/ml of TGF-β and conditioned media was collected as described in the Materials and Methods. Results are the mean ± SE (*p < 0.05 and **p < 0.01 vs. unstimulated control n = 3 per group). Similar results were obtained in three separate experiments. Data are shown for one experiment.
DISCUSSION
Osteolytic bone metastases cause significant morbidity in patients with breast cancer. Recently, such lesions have been shown to be mediated, in part, by tumor production of the osteoclast-stimulating factor PTHrP. Osteoclastic bone resorption results in release of bone-derived growth factors from the mineralized bone matrix.8 These growth factors have chemotactic properties for breast cancer cells and may contribute to the “homing” or chemotaxis of breast cancer cells to the bone. In the present study, we have explored the role of endogenous motility, as opposed to chemotaxis, in the establishment of osteolytic bone metastases in vivo by comparing the ability of MDA-MB-231 cells exhibiting low or elevated in vitro cell motility.
These data suggest that MDA-MB-231 cells capable of performing elevated cell motility associated with low hsp27 expression formed more osteolytic bone metastases. This conclusion was upheld by the fact that mice inoculated with highly motile cells developed more and larger osteolytic lesions as assessed radiographically and histomorphometrically. In addition, mice inoculated with highly motile cells became paraplegic earlier than the mice inoculated with low motility cells. These data support the hypothesis that motility plays a role in the formation of bone metastases and suggests that highly motile breast cancer cells may have an advantage to at least establish themselves in the bone environment as metastases. Furthermore, the mechanism of cell motility appears to be independent of the osteolytic capacity of the tumor cells studied here, as osteoclast number per square millimeter of tumor–bone interface and PTHrP production were similar in all the groups. The establishment of the less motile cells in the bone environment is retarded in our model, but whether this equates to only a slowing of the formation and expansion of metastases and not a complete prevention of metastasis in not understand at present since the animals were all sacrificed at the same time.
Evidence supporting the concept that the release of growth factors by osteoclastic bone resorption contributes to the chemotaxis of breast cancer cells to the bone is demonstrated by studies using bisphosphonates, potent inhibitors of bone resorption, for the treatment of bone metastases. Sasaki et al.13 have recently shown that bisphosphonate residronate reduced the metastatic human breast cancer burden in the bone of nude mice. Other published studies suggest that many cellular activities along with motility are important in bone metastases. Overexpression of E-cadherin, a cell-to-cell adhesion molecule, in MDA-MB-231 cells suppressed the development of osteolytic bone metastases4 and this may be explained in part by the fact that E-cadherin decreased cell motility in vitro,16 but E-cadherin also promotes cell–cell interactions which hinder the passage of single cells through the fenestra of small blood vessels found in the bone marrow,17 so the contribution of motility to the metastatic process in these studies is not clearly defined. It was also demonstrated by Nakai et al.18 that a synthetic antagonist to laminin inhibited the formation of osteolytic metastases by human melanoma cells in nude mice. A negative effect on cell motility may also explain this inhibition since laminin antagonists have been shown to block in vitro cell motility due to an altered cell adhesion mediated by some specific integrins.19, 20 Thus, there is a large amount of data supporting an important role of some matrix constituants of the basement membrane in facilitating tumor cell migration and invasion at the bone site. Other cellular activities, such as matrix metalloproteinase secretion, or expression of inhibitors of metalloproteinases, are also undoubtably important for the formation of bone metastases by breast and prostate cancer cells,21, 22 and we are currently examing these latter activities in our transfectatants.
The hsp27-overexpressing clones 19 and 12(2) used in this study have been previously shown to exhibit increased invasiveness, adhesion, and proliferation,11 implicating a positive association between hsp27 overexpression and these cellular activities. It appears from the present study that decreased motility associated with hsp27 overexpression, but not these other biological parameters, may be an important determinant for these cells to metastasize to the bone, at least in this model. Thus, the increased adhesive and invasive properties of these cells does not overcome the disadvantage of reduced cell motility for metastasis to the bone. We also know that breast cancer cells overexpressing hsp27 are resistant to certain chemotherapeutic agents23 and that hsp27 is a mediator of confluence-dependent resistance to anticancer drugs in colorectal cancer cell lines,24 but it does not seem that proliferation is an important parameter for hsp27-associated drug resistance, since cellular proliferation increased in one study23 but decreased in the other as a consequence of hsp27 overexpression.24 The importance and contribution of resistance in hsp27 overexpressing cells to the metastatic phenotype is not known.
In summary, the data presented here demonstrate that cell motility is an important step in the establishment of osteolytic bone metastases and that it is independent of the osteolytic capacity of the breast cancer cells. Mechanisms directed at controlling cell motility may therefore constitute new targets to prevent the formation of such metastatic lesions. This new therapeutic strategy deserves further investigation in an effort to decrease the morbidity associated with breast cancer metastases to the bone.
Acknowledgements
This study was supported by National Institutes of Health grants CA585183 and CA54174 to S.W.A.F., AR01899 to T.A.G., and a Susan G. Komen Foundation Postdoctoral Fellowship to P.L.




