Development and Characterization of a Human In Vitro Resorption Assay: Demonstration of Utility Using Novel Antiresorptive Agents
Abstract
A human in vitro resorption assay has been developed using osteoclastoma-derived osteoclasts and used to evaluate novel antiresorptive agents including antagonists of the αvβ3 integrin, and inhibitors of cathepsin K and the osteoclast ATPase. The potency of novel compounds in the in vitro resorption assay correlates with functional assays for each class of inhibitor: the human αvβ3-mediated cell adhesion assay for the vitronectin receptor antagonists (r2 = 0.82), the chick osteoclast vacuolar ATPase enzyme assay for the H+-ATPase inhibitors (r2 = 0.77) and the recombinant human cathepsin K enzyme assay for the cathepsin K inhibitors (r2 = 0.80). Cell suspensions, rich in osteoclasts, are prepared by collagenase digestion of the tumor tissue. These cells can be stored long-term in liquid nitrogen and upon thawing maintain their bone-resorbing phenotype. The cryopreserved cells can be cultured on bovine cortical bone for 24–48 h and resorption can be measured by either confocal microscopy or biochemical assays. The resorptive activity of osteoclasts derived from a number of tumors can be inhibited reproducibly using a number of mechanistically unique antiresorptive compounds. In addition, the measurement of resorption pits by laser confocal microscopy correlates with the release of type I collagen C-telopeptides or N-telopeptides, as measured by enzyme-linked immunosorbent assay. Resorption can be measured reproducibly using a 48-h incubation of osteoclasts on bone slices, or a 24-h incubation with bone particles. This in vitro human osteoclast resorption assay provides a robust system for the evaluation of inhibitors of osteoclastic function that may be developed for the treatment of metabolic bone diseases such as osteoporosis.
INTRODUCTION
OSTEOCLASTS ARE MULTINUCLEATED CELLS of hematopoietic origin that are responsible for the resorption of bone.1, 2 In the normal human adult skeleton, these cells are rare and reside on calcified surfaces within the medullary cavity. Consequently, it is extremely difficult to reproducibly isolate large numbers of human osteoclasts for study in vitro. Investigators have isolated and characterized disaggregated osteoclasts from neonatal rodents (reviewed in Murrills et al.3) and embryonic chickens,4 where osteoclasts are relatively abundant. Although use of these cells provides valuable information, significant species differences can exist between cells or, at the molecular level, between enzymes or receptors. Therefore, when considering the development of compounds, for targeting human osteoclast-mediated bone resorption, it is necessary to develop a model system that utilizes human cells.
Important information regarding human osteoclast biology has been obtained from osteoclasts derived from long-term marrow cultures.5 However, these methods are unable to provide sufficient numbers of cells for routine screening of potential antiresorptive agents. In addition, although there has been progress in the identification of human osteoclast-like cell lines,6-8 none have been reported that could be used routinely in resorption assays. Consequently, the vast majority of reports describing osteoclastic resorption involve assays that utilize osteoclasts of mouse,9 rat,10 rabbit,11 or chicken12 origin.
In this study, we describe the isolation, characterization, and use of human osteoclastoma-derived osteoclasts in resorption assays for the evaluation of antiresorptive agents. We and others have previously demonstrated that osteoclastoma-derived giant cells are phenotypically indistinguishable from osteoclasts.13, 14 These cells are multinucleated and express tartrate-resistant acid phosphatase (TRAP) activity, vacuolar ATPase activity,15 calcitonin receptors,16, 17 vitronectin receptors,18 and cathepsin K,19 and they possess the ability to resorb pits in bone or dentine. We demonstrate here that these human osteoclasts can be reproducibly isolated and can maintain their bone-resorbing phenotype even after long-term freezing. Bone resorption can be measured using either confocal microscopy to measure resorption pits or biochemical assays, which can be utilized to quantitate osteoclast-mediated type I collagen degradation.20, 21 Significantly, these cells can be used reproducibly to characterize compounds that inhibit mechanisms that are crucial for efficient osteoclast-mediated resorption including cell adhesion to bone,22 resorption pit acidification,23 or matrix degradation.24, 25
MATERIALS AND METHODS
Tissue culture media and media supplements were from GIBCO Laboratories (Grand Island, NY, U.S.A.). Tissue culture plastics were supplied by Falcon (Lincoln Park, NJ, U.S.A.). Dynabeads were obtained from Dynal, Inc. (Great Neck, NY, U.S.A.). Cryovials and flat-bottom Nunclon 96-well plates were from Nunc (Roskilde, Denmark). Type I collagenase (from Clostridium histolyticum) and all general chemicals were from Sigma Chemicals (St. Louis, MO, U.S.A.). The Isomet low-speed saw was purchased from Buehler (Lake Bluff, IL, U.S.A.). Bovine cortical bone particles were obtained from Pel-Freeze Biologicals (Rogers, AR, U.S.A.). Sieves for grading bone particles were obtained from Newark Wire Cloth Company (Newark, NJ, U.S.A.). The C-telopeptide resorption enzyme-linked immunosorbent assay (ELISA) kits were supplied by Osteometer A/S (Rodovre, Denmark) and the N-telopeptide ELISAs were from Ostex (Seattle, WA, U.S.A.). The MRX-HD plate reader was from Dynatech Laboratories (Chantilly, VA, U.S.A.). The Olympus Vanox AHBT3–3 fluorescence microscope was purchased from Hitech Instruments, Inc. (Edgemont, PA, U.S.A.). The laser 1LM21 laser confocal microscope was supplied by Nikon/Lasertec Corporation (Melville, NY, U.S.A.). Inhibitor compounds were synthesized at SmithKline Beecham.
Preparation of cells from osteoclastoma tissue
All osteoclastoma tissue was obtained by informed consent at the time of surgery. The tissue was digested with 3 mg/ml (w/v) of bacterial type I collagenase in serum-free RPMI-1640 medium. After replacement of the collagenase solution with 10 ml of RPMI medium supplemented with 10% fetal bovine serum (FBS), the tissue was gently homogenized. This process was repeated until no further osteoclasts were released from the tissue. The number of viable osteoclasts was adjusted to 1 × 105 cells/ml in freezing medium (40% RPMI medium, 50% FBS, and 10% dimethylsulfoxide), and 1 ml aliquots of the suspension were stored in liquid nitrogen until required for a resorption assay.
Preparation of bovine bone slices
Whole bovine femurs were prepared as previously described.3 Cortical bone slices (6 × 6 × 0.8 mm) were cut from previously prepared rectangular rods on a low-speed saw and stored in 70% ethanol until required. Prior to use in a resorption assay, the slices were sonicated for 5 minutes in distilled water and washed for 2 h in two changes of fresh distilled water. The slices were resterilized in fresh 70% ethanol and placed into wells of a 48-well tissue culture plate. Following washing, the slices were incubated overnight at 37°C prior to use in resorption assays.
Preparation of bovine cortical bone particles
Pulverized bone particles were bulk washed in numerous changes of distilled water, followed by four or five washes in 70% ethanol, after which the particles were left to dry under a biological hood. Particles of ∼500 μm were collected by sequential sieving. Prior to use in a resorption assay, approximately ∼30 mg (dry weight) of the particles were placed into wells of a flat-bottomed 96-well plate. After washing, the bone particles were incubated overnight at 37°C and then used in a resorption assay.
Resorption assay
Vials of frozen osteoclast-rich cell suspensions were rapidly thawed at 37°C. The cells were transferred into RPMI/10% FBS and pelleted by centrifugation. The cells were resuspended in 10 ml of cold medium containing anti-human HLA Class II antigen-coated magnetic beads. These antigens are not expressed by osteoclasts but are expressed on a number of cell types, including cells of the myeloid/monocytic lineages, activated T-cells, and certain epithelial cells. The beads (3 beads/mononuclear cell) were incubated with the cell suspension for 30 minutes on ice, and the bound mononuclear cells were removed from the osteoclasts by immobilizing the beads on a magnet. The osteoclast-rich suspension was pipetted into a fresh 50 ml centrifuge tube, and the bead-coated cell pellet was washed 10 times in cold medium. After each wash, the medium was removed from the immobilized beads and placed into the tube containing the osteoclast-rich suspension. The enriched osteoclast population of cells was then pelleted by centrifugation at 400g for 5 minutes and resuspended in fresh medium.
Forty-eight hour bone slice assay:
The bone slice assay was based on the methods described by Boyde and Jones26 and Chambers et al.11 Viable osteoclasts were enumerated in a counting chamber using fluorescein diacetate. The density of osteoclasts was adjusted to 2 × 103 cells/ml with fresh resorption medium, and the cells were pelleted by centrifugation and resuspended with or without inhibitors or dimethylsulfoxide vehicle. For inhibitor evaluation, the cells were preincubated with the compounds for 30 minutes at 37°C and applied to the previously prepared bovine bone slices in wells of a 48-well tissue culture plate (0.5 ml/well, each dose tested in triplicate). After allowing the cells to settle for 2 h at 37°C, the bone slices were washed in six changes of warm, sterile PBS and then placed into wells of a fresh 48-well plate, containing the appropriate concentration of compound or vehicle control. The cells were incubated on the bone slices for a further 48 h at 37°C, and the culture supernatants were collected into 1 ml cryotubes and stored at 4°C until required for testing in the type I collagen telopeptide ELISAs.
To determine pit volumes, after incubation for 48 h with cells, the bone slices were sonicated for 5 minutes in 1 M ammonium hydroxide and then washed in distilled water. The slices were air dried and three-dimensional measurements of resorption pits were made using a video-rate reflection confocal laser scanning microscope and dedicated software. Volume data were obtained from the map image which involved tracing a line around the pits. The software then determined the volumes below the area enclosed within the traces.27
Twenty-four hour bone particle assay:
This assay was performed as above except that the cells were cultured on the bone particles in the presence of compounds for 24 h and at the end of the culture period the supernatants were removed and clarified by centrifugation prior to storage at 4°C.
Resorption ELISAs
The competitive ELISAs for the measurement of C-telopeptides20 and N-telopeptides21 were performed according to the manufacturer's protocols. The results were obtained from both ELISAs by measuring absorbance at 450 nm. To determine the extent of resorption in individual samples, the results for both ELISAs were expressed as a percentage inhibition of resorption compared with supernatants derived from osteoclasts cultured in the absence of inhibitors.
Inhibition of αvβ3-mediated cell adhesion
To determine if potent αvβ3 antagonists could inhibit αvβ3-mediated cell adhesion, an adhesion assay was established using human embryonic kidney (HEK)-293 cells cotransfected with recombinant human αv and β3.28 A 96-well plate assay was performed, as previously described,28 except that human vitronectin was used as the substrate (1.5 μg/ml in RPMI medium). The potency of the compounds was evaluated using a multidose titration to determine an IC50 value.
Chicken osteoclast ATPase assay
Chicken osteoclast membranes were prepared as previously described29 and bafilomycin-sensitive ATPase activity was measured23 using the malachite green colorimetric assay.30
Cathepsin K enzyme assay
The Ki apparents of potential cathepsin K inhibitors were determined using recombinant human cathepsin K, as previously described.24
RESULTS
Characterization of osteoclastoma-derived osteoclasts
Our studies confirmed that the osteoclastoma-derived giant cells expressed an osteoclastic phenotype. These cells are multinucleated (Fig. 1A), express TRAP activity (Fig. 1B) and possess the ability to resorb pits in bone or dentine (Figs. 1C and 1D).
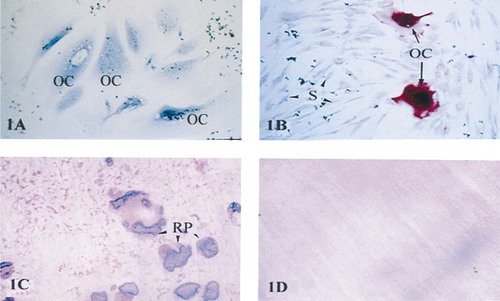
Phenotypic characterization of osteoclastoma-derived giant cells. Osteoclastoma-derived osteoclasts cultured for 24 h in RPMI medium supplemented with 10% FBS were fixed in methanol and stained for multinuclearity, using a modified Rowmanowsky stain (A) and TRAP activity (B). OC, osteoclast; S, stromal cells. Original magnification ×10. Osteoclast-rich cell suspensions were also seeded onto sperm whale dentine slices and incubated for 24 h in the same medium as (A) and (B). The medium was aspirated and the cells were removed from the surface of the dentine by sonication. Resorption lacunae were stained with toluidine blue. Numerous resorption pits (RP) can be seen on the surface of the dentine (C) compared with the control that was cultured in the absence of cells (D). Original magnification ×10.
Use of osteoclastoma-derived osteoclasts in resorption assays
Reproducible pit formation could be achieved by the osteoclastoma-derived osteoclasts. To determine whether this activity could be inhibited, osteoclastoma-derived cells were treated with antagonists of the vitronectin receptor (αvβ3). Since this receptor is intimately involved in the attachment of the osteoclasts to the bone surface,32 it was surmised that αvβ3 antagonists would block resorption. The inhibition of resorption was quantitated in these experiments by measuring the pit volumes using laser confocal microscopy. Echistatin, a snake venom–derived peptide vitronectin receptor antagonist,33 demonstrated potent and dose-dependent inhibition of human osteoclast resorption with an IC50 of ∼25 nM (Fig. 2). A small molecule αvβ3 antagonist also demonstrated dose-dependent inhibition of osteoclast resorption, measuring pit volume, with an IC50 of 8 μM (Fig. 2). These compounds inhibited αvβ3-mediated cell adhesion with IC50's of 3 nM and 470 nM, respectively (data not shown).
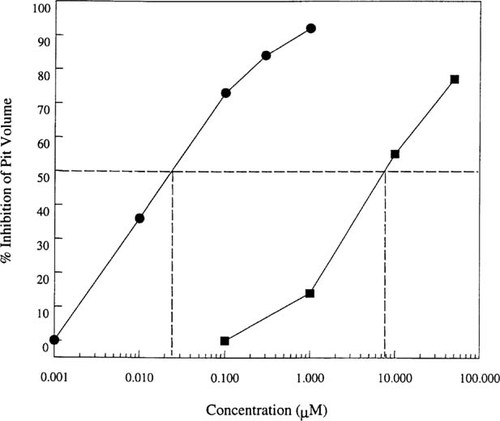
Vitronectin receptor (αvβ3) antagonists inhibit human osteoclast resorption. Osteoclasts were seeded onto bovine bone slices in the presence or absence of various concentrations of αvβ3 antagonists. The volumes of the resorption pits were measured by laser confocal microscopy and the results are expressed as percentage inhibition compared with an uninhibited control. The snake venom peptide, echistatin (circles), potently inhibited resorption with an IC50 of ∼20 nM. A small molecule nonpeptide antagonist of αvβ3 (squares) was also able to inhibit resorption with an IC50 of ∼8 μM (IC50 values are indicated by the broken lines).
Comparison of the biochemical readouts with the measurement of osteoclast pit area and volume
Classically, bone resorption has been measured by quantitating pit number, area, or volume. Since this is extremely time consuming, we evaluated the potential utility of biochemical readouts of resorption. Four cathepsin K inhibitors were screened at 5 μM in the 48 h bone slice assay. The number, area, and volume of the resorption lacunae were measured by laser confocal microscopy. As a comparison, the four compounds were also screened in the 24 h, 96-well bone particle assay and the type I collagen C-telopeptide (CTX) and N-telopeptide (NTX) fragments of type I collagen were quantitated by ELISA. Each compound was tested at six dose points, ranging from 3 μM down to 0.01 μM in half-log doses. The results were calculated as percentage inhibition against an uninhibited vehicle control. None of the compounds had an effect on pit number (data not shown) and only SB 240314 demonstrated significant inhibition in resorbed area compared with the vehicle control (Fig. 3A). In contrast, there was a potency-dependent inhibition of pit volume with these compounds, i.e., the most potent inhibitor was the most effective at reducing pit volume (Table 1 and Fig. 3B). Furthermore, the rank order of this inhibition in pit volume correlated strongly with the CTX and NTX ELISA readouts (Table 1). SB 270022 demonstrated no inhibition of resorbed pit volume (Fig. 3B) and was inactive in both the ELISAs up to 3 μM (Table 1).
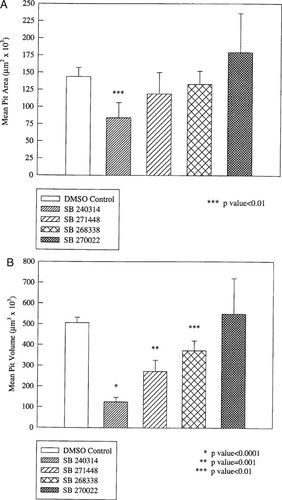
The inhibition of resorption pit area and volume by nonpeptide, small molecule inhibitors of cathepsin K. A bone slice assay was performed using inhibitors of human cathepsin K at a concentration of 5 μM. The area and volume of the resorption pits were measured by laser confocal microscopy. An irreversible inhibitor of cathepsin K (SB 240314), demonstrated significant inhibition of resorbed area, as compared with a vehicle control, whereas the reversible inhibitors SB 271448, SB 268338, and SB 270022 had no effect on pit area (A). In contrast, potency dependent inhibition of pit volume was observed with several of the compounds (B).
Reproducibility of the human osteoclast resorption assay
Osteoclast-rich cell preparations that had been stored in liquid nitrogen were treated with inhibitors from several unique classes of antiresorptives (Table 2). Multiple assays were performed on cells derived from a number of different tumors. SB 223245, an αvβ3 antagonist,34 was tested a total of 43 times against cells derived from five tumors. SB 230056, an ATPase inhibitor, was tested 22 times against cells from three tumors, and SB 240314, a cathepsin K inhibitor17 was tested 100 times against cells from five tumors. Even though this assay utilizes primary human cells from several different individuals, inhibition of resorption is reproducible (1.2- to 5.5-fold difference in IC50's for αvβ3 antagonists, 1.1- to 1.3-fold for ATPase inhibitors and 1.3- to 2.9-fold for cathepsin K inhibitors [Table 2]).
Correlation between resorption and other primary and secondary assays for each class of inhibitor
A selection of inhibitors from each of the three classes of antiresorptives was tested in the resorption assay, and their IC50 results were compared with the data generated from their respective functional assays. Eleven αvβ3 antagonists were screened in the HEK cell adhesion assay. Activities of these inhibitors in this assay were compared with their activity in the human osteoclast resorption assay (Fig. 4A). There was a correlation between these two assays (r2 = 0.82, p = 0.058) indicating that activity in the primary cell adhesion assay translates into antiresorptive activity in the human osteoclast resorption assay. Likewise, a correlation was also found when comparing activity of osteoclast vacuolar-ATPase inhibitors in the enzyme and resorption assays (r2 = 0.77, p = 0.004; Fig. 4B. Finally, a correlation was observed when the activity of a number of cathepsin K inhibitors was compared in the recombinant enzyme assay and the resorption assay (r2 = 0.80, p = 0.028; Fig. 4C).
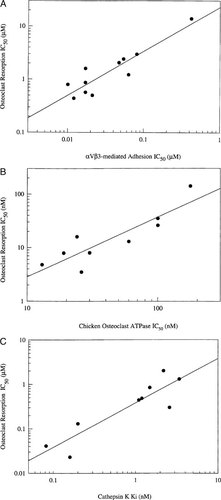
Biochemical readout of resorption correlates with primary and secondary assays for vitronectin receptor antagonists, vacuolar ATPase inhibitors, and cathepsin K inhibitors. Inhibition of osteoclast-mediated bone resorption was compared with inhibition of αvβ3-mediated cell adhesion with αvβ3 antagonists (A), to chicken ATPase activity (B), or recombinant human cathepsin K activity (C). p values were calculated using the paired t-test.
DISCUSSION
In vitro assays of osteoclast-mediated bone resorption have been described in several species. In this report, we have described such a cell source derived from human osteoclastoma. Using a combination of protease digestion, homogenization of the solid tumor, and negative selection of osteoclasts, we were able to obtain a source of human osteoclasts that performed reproducibly in bone resorption assays. This study confirms previous data showing that human osteoclastoma-derived giant cells are phenotypically indistinguishable from osteoclasts.14 Furthermore, we have demonstrated that these cells can be stored long-term in liquid nitrogen and used reproducibly in resorption assays. The assay is unique in that it provides a screening assay for studying the effects of inhibitors on human osteoclast resorption, thus eliminating any interspecies problems that may arise from screening inhibitors against rodent and avian osteoclasts. Recently, Breuil et al.35 developed a complimentary assay in which they generate human osteoclasts in vitro using human peripheral blood. It will be interesting to compare the activity of antiresorptive agents on these cells with the mature osteoclastoma-derived osteoclasts. Our data show that the assay can be configured in three ways: a standard format to enable the direct measurement of pit formation on bone slices, using laser confocal microscopy; a bone slice assay using the measurement of CTX by ELISA as the readout; and a 96-well plate format, using bone particles as the substrate and the measurement of CTX and NTX by ELISA as the readout. The biggest advantages of the third option compared with the second, in our hands, are higher throughput (96-well vs. 48-well) and a shorter incubation time to obtain a significant resorptive signal (24 h vs. 48 h).
The physical measurement of osteoclast pits by light microscopy or scanning electron microscopy on dentine or bone slices has been the classical approach for determining osteoclastic activity. Although investigators have reported osteoclast pit number and/or area as readouts of bone resorption, it is widely accepted that the measurement of pit volume provides the most sensitive and meaningful parameter of resorptive activity.36 We have previously reported data using laser-confocal microscopy to measure pit volumes in determining the antiresorptive activity of αvβ3 antagonists.37 However, this approach is very time consuming and does not lend itself to the evaluation of a large number of agents. Consequently, a major advance in developing the assay as a tool for screening antiresorptive compounds was the ability to adapt it to a 96-well format, using bovine or human cortical bone particles as the substrate, and using the measurement of type I collagen degradation products as surrogate markers of in vitro resorptive activity. We and others have shown that both the CTX20 and NTX21 collagen degradation products are appropriate markers for measuring resorption and its inhibition. Recent data from Atley et al.38 demonstrated that the NTX degradation product of type I collagen is specifically cleaved by cathepsin K. These data suggest that the NTX assay could be more appropriate for screening cathepsin K inhibitors than the CTX assay. However, in our studies, the activity of the cathepsin K inhibitors was very similar in both assays and correlated with the pit volume data generated by laser confocal microscopy. This study further demonstrated the lack of sensitivity of pit area or pit number as measures of bone resorption.
This resorption assay has been used to confirm the importance of three mechanisms in human osteoclast resorption. Significantly, it has also allowed us to develop potent inhibitors of these mechanisms to block the resorptive process. It has previously been demonstrated that osteoclast attachment to bone is mediated by the αvβ3 receptor18 and that the subsequent acidification of the resorptive microenvironment and the dissolution of bone mineral is under the control of the vacuolar H+-ATPase.15, 39 Finally, the degradation of the demineralized matrix is largely mediated by cathepsin K, a cysteine protease that is abundantly expressed in osteoclasts.19 We have shown in this study that targeted inhibition of αvβ3,22 vacuolar H+-ATPase,23 and cathepsin K,24, 25, 31 using both peptide and rationally designed small molecule peptidomimetic inhibitors, will inhibit the resorptive process in human osteoclasts. These data indicate that inhibition of any number of osteoclastic activities, including attachment to bone, acidification of the resorption lacunae, demineralization, or organic matrix degradation can result in inhibition of bone resorption.
Although there is good correlation between the resorption assay and the other functional assays, there is not 100% correlation in potency, e.g., 100- to 1000-fold difference between the Ki apparents of the inhibitors in the cathepsin K enzyme assay compared with the IC50's in the resorption assay. The reason for this shift in potency may be differences in the assay conditions; the Ki data are generated against purified recombinant enzyme while the resorption data are generated from a complex cell-based assay.
In conclusion, we describe in this study a human osteoclast-based resorption assay that utilizes osteoclasts isolated from osteoclastoma tissues. Furthermore, these osteoclasts can be stored indefinitely in liquid nitrogen and can perform reproducibly to screen large numbers of potential antiresorptive agents that target different mechanisms involved in the resorptive process. This assay has provided an important in vitro assay for the identification of candidate antiresorptive drugs for the treatment of metabolic bone diseases such as osteoporosis.
Acknowledgements
We thank Drs. Alison Badger and John Emery for critical review of the manuscript. We also thank Dr. Richard Lackman of the Jefferson Hospital, Philadelphia, PA, U.S.A., for supplying the osteoclastoma tissues. These studies were funded by SmithKline Beecham Pharmaceuticals.






