24,25-Dihydroxyvitamin D3 Suppresses the Rapid Actions of 1,25-Dihydroxyvitamin D3 and Parathyroid Hormone on Calcium Transport in Chick Intestine
Abstract
Studies were undertaken to determine whether 24,25-dihydroxyvitamin D3 (24,25(OH)2D3) modulates the rapid effects of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) and parathyroid hormone (PTH) on calcium transport in the perfused chick intestine. Perfusion with control media resulted in a transport ratio (treated/average basal) of 1.07 ± 0.06 at t = 40 minutes, while perfusion with 65, 130, 300, or 650 pM 1,25(OH)2D3 yielded ratios of 1.92 ± 0.23, 2.6 ± 0.4, 2.8 ± 0.08, and 3.34 ± 0.37, respectively. Simultaneous perfusion with each of these doses and 6.5 nM 24,25(OH)2D3 reduced treated/average basal ratios to ∼1.4 after 40 minutes of perfusion. Vascular perfusion with 65 pM bovine PTH [bPTH(1–34)] stimulated intestinal calcium transport ratios to 3.0 ± 0.5 after 40 minutes, while the inclusion of 6.5 nM 24,25(OH)2D3 reduced ratios at this time point to 0.56 ± 0.19. To investigate the effect of these agents on signal transduction, isolated intestinal cells were monitored for intracellular calcium changes using the indicator dye fura-2. After establishing a stable baseline, addition of 130 pM 1,25(OH)2D3 induced rapid calcium oscillations. Intestinal cells exposed to 6.5 nM 24,25(OH)2D3 also exhibited rapid oscillations in fluorescence, which were not further altered by subsequent addition of 1,25(OH)2D3. Incubation of isolated cells with 130 pM 1,25(OH)2D3 was found to increase protein kinase C (PKC) activity within 5 minutes, and protein kinase A (PKA) activity within 7 minutes. Exposure of cells to 65 pM bPTH(1–34) had minimal effect on PKC activity, but resulted in pronounced increases in PKA activity. Stimulation of protein kinases by either secosteroid or peptide hormone was inhibited in the presence of 6.5 nM 24,25(OH)2D3. It is concluded that 24,25(OH)2D3 may exert endocrine actions on intestine.
INTRODUCTION
VITAMIN D IS METABOLIZED in the body to 25-hydroxyvitamin D3in the liver, which in turn is metabolized to more polar metabolites: 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), a hormonally active secosteroid, and 24,25-dihydroxyvitamin D3 (24,25(OH)2D3), a metabolite once thought to be an inactivation product, but which is beginning to be appreciated as a hormone as well.
Early studies suggested that both 1,25(OH)2D3 and 24,25(OH)2D3 are required for normal chick hatchability1 and bone formation.2 Indeed, most studies on the effects of 24,25(OH)2D3 have been conducted in bone,3-5 including confirmation that the metabolite is required for fracture healing.6-8 In clinical studies of patients with X-linked hypophosphatemic rickets, 24,25(OH)2D3 has been found to ameliorate hyperparathyroidism, allowing greater bone mineralization9
Since 1,25(OH)2D3 is synthesized under conditions of vitamin D, calcium, and phosphate insufficiency, while 24,25(OH)2D3 is produced when 1,25(OH)2D3, calcium, and phosphate are sufficient, it is possible that 24,25(OH)2D3 is an endogenous antagonist of 1,25(OH)2D3 action. At the cellular level, 24,25(OH)2D3 has been found to inhibit the rapid non-nuclear effects of 1,25(OH)2D3 on opening calcium channels is osteoblasts and osteosarcoma cells.10-12 Sundell and Bjornsson13 have reported that 24,25(OH)2D3 acutely decreases intestinal calcium transport in Atlantic cod, a fish confronted with an overabundance of environmental calcium. In chick intestine, 24,25(OH)2D3 has been reported to inhibit the acute, non-nuclear effects of 1,25(OH)2D3 on phosphate transport,14 while stimulation of phosphate transport by parathyroid hormone (PTH) was unaffected.15 In the current work, the effect of 24,25(OH)2D3 on the acute stimulation of calcium transport by either 1,25(OH)2D3 or PTH was assessed, as well as modulation of signal transduction pathways activated by the stimulatory hormones.
MATERIALS AND METHODS
Animals and surgical procedures
White Leghorn cockerels were raised on a vitamin D–sufficient diet for 5–8 weeks prior to experimentation. All protocols were approved by the Institutional Oversight Committee on the Care and Use of Animals.
For perfusion studies, procedures were as described earlier.14 Briefly, animals were anesthetized, the duodenal loop surgically exposed, the celiac vein cannulated, and perfusion with Gey's balanced salt solution (GBSS) begun. After removal of the loop, the juxtaposed vein was cannulated for collection of the venous effluent, and the lumen cannulated for perfusion with 1 μCi/ml of45CaCl2 in GBSS lacking bicarbonate and glucose. Basal transport occurred for the first 20 minutes, followed by a 40-minute treated phase in which vascular perfusion was continued with control media or with the indicated levels of either 1,25(OH)2D3, bovine parathyroid hormone (bPTH)(1–34) (Sigma Chemical Co., St. Louis, MO, U.S.A.), or hormone in the presence of 24,25(OH)2D3.
Cell isolation and incubation protocols for protein kinase measurements
Intestinal epithelial cells from two duodena per experiment were isolated by citrate chelation16 and resuspended in GBSS (adjusted to pH 7.3). For time course studies on the activation of protein kinase C (PKC) or protein kinase A (PKA), 20-ml cell suspensions were divided between two polypropylene beakers, each containing 10 ml of GBSS and stirring fleas for gentle mixing on 6 × 6 magnetic stirrers (Cole Parmer, Vernon Hills, IL, U.S.A.). Under these conditions >90% of the cells remain viable for 40 minutes, as judged by trypan blue exclusion. At zero time, duplicate 100 μl samples (∼106 cells) were removed from each beaker to microfuge tubes, and the remaining suspensions treated with either vehicle (0.05% ethanol for vitamin D metabolites) or 65 pM bPTH(3–34) (Sigma) for controls, while suspensions exposed to active hormones received either 130 pM 1,25(OH)2D3 or 65 pM bPTH(1–34) (final concentrations). Duplicate 100 μl samples were subsequently removed at 1, 3, 5, 7, and 10 minutes after additions and held on ice. The cell samples were then centrifuged at 500g for 5 minutes, the supernatants decanted, and the interior walls of the tubes swabbed to remove any adherent liquid.
For single time point studies, isolated cells from two duodena were resuspended in 40 ml of GBSS, pH 7.3, and 100 μl added to microfuge tubes containing an additional 100 μl of buffer. At zero time, 200 μl of media containing the appropriate control substances, 130 pM 1,25(OH)2D3 with or without 6.5 nM 24,25(OH)2D3, or 65 pM bPTH(1–34) with or without 6.5 nM 24,25(OH)2D3, were added. After an additional 5–7 minutes incubation (see below), cells were centrifuged as described above.
Determination of fura-2 fluorescence
Cell pellets were resuspended (20 ml/duodenum) in phosphate-buffered saline (PBS) containing 0.3% bovine serum albumin, 1 mM EDTA, 5.55 mM glucose. Cell suspensions were then incubated with Pluronic acid and fura-2 as described elsewhere17 for 20 minutes on ice. Then cells were diluted to 40 ml with PBS, centrifuged, and resuspended in 20 ml of PBS. A 500-μl aliquot was combined with sufficient CaCl2 to yield a final concentration of 1 mM, and 200 μl aliquots taken for microscopic determination of fluorescence in the absence or presence of hormones.
Analytical determinations
Protein was determined using the Bradford dye from Bio-Rad (Hercules, CA, U.S.A.). PKC and PKA were determined according to instructions packaged with the commercially available kits (Life Technologies-GIBCO, Waverly, MA, U.S.A.). Calculations of activities were also as recommended by the kit manufacturers). Radioactively labeled ATP was purchased from New England Nuclear (Boston, MA, U.S.A.).
RESULTS
The first series of experiments using the perfused duodenal loop system were undertaken to determine whether 24,25(OH)2D3 altered a physiological response, i.e., enhanced intestinal calcium transport in response to 1,25(OH)2D3. A single, physiological concentration18 of 6.5 nM 24,25(OH)2D3 was tested with increasing concentrations of 1,25(OH)2D3. Earlier reports14, 19 have indicated that ≤ 10 nM 24,25(OH)2D3 has no effect on either calcium or phosphate transport, relative to duodena perfused with control media. As illustrated in Fig. 1, increasing concentrations of 1,25(OH)2D3 in the vascular perfusate resulted in increasing levels of45Ca in the venous effluent, relative to controls (n = 4): after 40 minutes of perfusion, treated/average basal levels were (mean ± SEM) 1.92 ± 0.23 for 65 pM 1,25(OH)2D3 (n = 9; Fig. 1A); 2.6 ± 0.4 for 130 pM 1,25(OH)2D3 (n = 3; Fig. 1B); 2.78 ± 0.08 for 300 pM 1,25(OH)2D3 (n = 3; Fig. 1C); and 3.34 ± 0.37 for 650 pM 1,25(OH)2D3 (n = 3, Fig. 1D). Simultaneous perfusion of duodena with 6.5 nM 24,25(OH)2D3 and any of the four concentrations of 1,25(OH)2D3 tested resulted in a decreased level of45Ca in the venous effluent, relative to 1,25(OH)2D3 alone (Figs. 1A–1D). In addition, it was evident that the inhibitory effect of 24,25(OH)2D3, relative to 1,25(OH)2D3 alone, only became apparent after 10 minutes of perfusion, and that the degree of inhibition was similar for all concentrations of 1,25(OH)2D3 tested. The similar degree of inhibition is more readily apparent in Fig. 2, in which the treated/average basal ratio of transport is plotted for the 40-minute time points. The t = 40 minutes ratios for duodena perfused with 6.5 nM 24,25(OH)2D3 and either 65, 130, 300, or 650 pM 1,25(OH)2D3 were (mean ± SEM) 1.4 ± 0.23 (n = 3), 1.43 ± 0.2 (n = 3), 1.67 ± 0.22 (n = 3), 1.4 ± 0.21 (n = 3), respectively. The statistical differences for transport at 130, 300, or 650 pM 1,25(OH)2D3 relative to transport in the presence of 24,25(OH)2D3 were P < 0.01, P < 0.02, and P < 0.05, respectively.
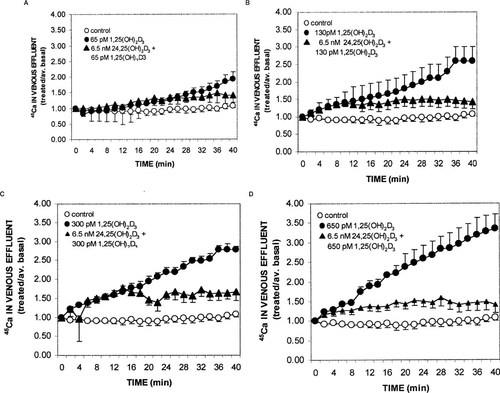
Effect of vascular perfusion with control media, or increasing concentrations of 1,25(OH)2D3 in the absence or presence of 6.5 nM 24,25(OH)2D3 on intestinal calcium transport. Chicks were raised to ∼500 g on a commercially available vitamin D–replete diet. Following anesthesia, the duodenal loops were cannulated through the celiac artery and excised; thereafter the celiac vein and lumenal apertures were cannulated. Vascular perfusion media contained GBSS and 0.125% bovine serum albumin and either vehicle or test substances in the indicated amounts. Lumenal perfusion media consisted of GBSS lacking bicarbonate and glucose, and containing 1 μCi/ml45CaCl2. Basal transport was assessed during the latter half of the 20-minute period, whereupon test substances were introduced and the venous effluent collected for an additional 40 minutes. Values represent mean ± SEM for four duodena perfused with control media, nine with 65 pM 1,25(OH)2D3 and three duodena per group for the remaining test situations.
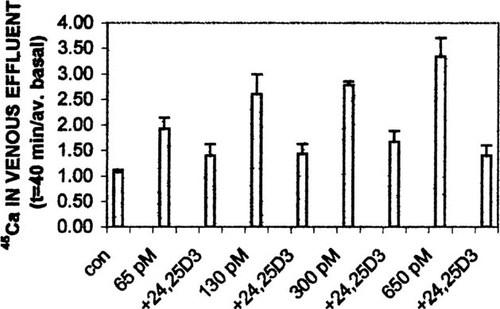
Summary of calcium transport at 40 minutes of perfusion. Data from Fig. 1 are replotted to allow comparison of calcium transport effect due to increasing concentrations of 1,25(OH)2D3 in the absence and presence of 6.5 nM 24,25(OH)2D3.
In another perfusion study, the effect of 6.5 nM 24,25(OH)2D3 on PTH-induced stimulation of intestinal calcium transport was analyzed. As shown in Fig. 3, 65 pM bPTH(1–34) enhanced the appearance of45Ca in the venous effluent to 3.0 ± 0.5 (n = 3) after 40 minutes of perfusion, while the presence of the vitamin D metabolite resulted in treated/average basal ratios that were 0.56 ± 0.19 (P ≈ 0.01). Thus, 24,25(OH)2D3 decreases or abolishes the stimulatory effects of both 1,25(OH)2D3 and PTH.
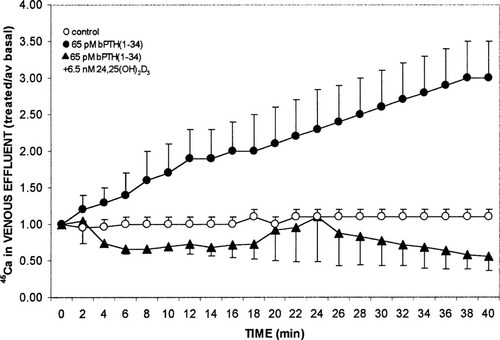
Effect of vascular perfusion with control media, or 65 pM bPTH(1–34) in the absence or presence of 6.5 nM 24,25(OH)2D3 on intestinal calcium transport. Procedures were as described in the legend to Fig. 1. Values represent mean ± SEM for three duodena per group.
Additional studies were undertaken to assess the effect of 24,25(OH)2D3 on selected signal transduction pathways that have been implicated in the rapid effects of 1,25(OH)2D3. Figure 4 depicts representative results for the effects of 130 pM 1,25(OH)2D3 in the absence or presence of 6.5 nM 24,25(OH)2D3, on free intracellular calcium as judged by the fluorescent indicator dye, fura-2. In the absence of additives, a stable baseline was achieved at a fluorescence ratio of ∼0.2 (Fig. 4) Each reading at 3-s intervals represents the average of ∼100 cells. Immediately following the addition of 1,25(OH)2D3, calcium oscillations appeared, characterized by spikes of fluorescence up to a ratio of 1, and declines to basal fluorescence levels (Fig. 4, top panel). Addition of 24,25(OH)2D3 to isolated chick intestinal cells also resulted in intense calcium spikes (Fig. 4, bottom panel, t = 60–100 s) interspersed with declines in fluorescence that were below basal levels. Addition of 130 pM 1,25(OH)2D3 (t = 109 s) did not noticeably alter the oscillatory behavior from that observed with 24,25(OH)2D3 alone (Fig. 4, bottom panel). The vehicle ethanol, in a range of 0.004–1%, final concentration, had no effect on fura-2 fluorescence.
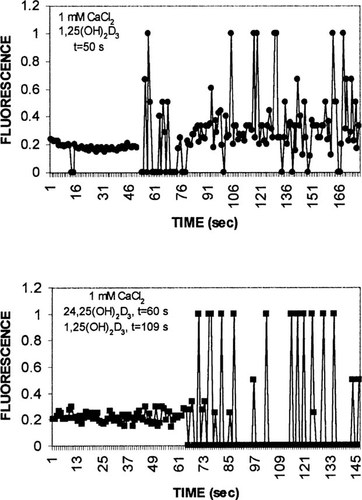
Effect of secosteroids on intracellular calcium as judged by Fura-2 fluorescence. Intestinal cells isolated from two chick duodena were loaded with Fura-2 for 20 minutes on ice. After washing and resuspension in PBS, media were made 1 mM in CaCl2. Two hundred microliters of suspension was placed on microscope slides for monitoring with a ×40 objective. (Top panel) 130 pM 1,25(OH)2D3 (final concentration) added at 50 s; (bottom panel) 6.5 nM 24,25(OH)2D3 (final concentration) added at 60 s, and 130 pM 1,25(OH)2D3 (final concentration) added at 109 s. Values represent representative results for six independent experiments.
The effects of the calcitropic hormones on PKA and PKC were also analyzed. Figure 5 illustrates the time course of PKC and PKA activities in response to treatment of isolated intestinal epithelial cells with 130 pM 1,25(OH)2D3. Stimulation of PKC activity to 3-fold of control levels was detected 5 minutes after addition of secosteroid and declined at 7–10 minutes after hormone (n = 3), while stimulation of PKA activity was maximal 7 minutes after 1,25(OH)2D3 (n = 3).
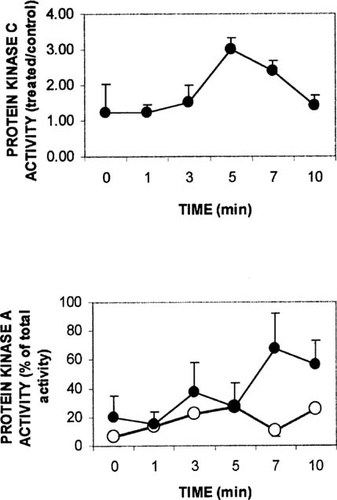
Time course of 1,25(OH)2D3 effect on PKC and PKA. Isolated intestinal cells were suspended in GBSS buffered to pH 7.3 and placed in each of two plastic beakers (containing stirring fleas) on magnetic stirrers. Aliquots were removed at zero time and the remaining suspension treated with vehicle (0.05% ethanol, final concentration) or 130 pM 1,25(OH)2D3. Aliquots were removed in duplicate at the indicated times, the cells harvested by centrifugation, homogenized, and assayed for protein kinase activity according to instructions in commercially available kits. (Lower panel) filled circle, hormone; open circle, vehicle. Values represent mean ± SEM for three independent experiments.
PKC activity was stimulated to a lesser degree by 65 pM bPTH(1–34) (Fig. 6, upper panel), while PKA exhibited a sustained increase in activity from 5–10 minutes after the peptide hormone (each, n = 2). The results of the time course studies suggested that the effect of 24,25(OH)2D3 on 1,25(OH)2D3 could best be assessed using PKC activity 5 minutes after hormone, and the effect on PTH activity could be assessed using PKA activity at 7 minutes after hormone.
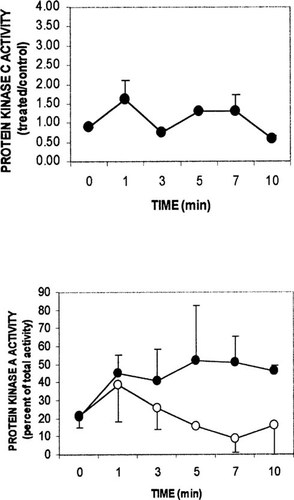
Time course of 65 pM bPTH(1–34) effect on PKC and PKA. Protocols were as described in the legend to Fig. 5. (Lower panel) filled circle, bPTH(1–34); open circle, bPTH(3–34). Values represent mean ± range for two independent experiments.
Figure 7 (upper panel) illustrates the results of three independent experiments in which isolated intestinal cells were incubated in the presence of the vehicle ethanol (0.05% final concentration), 130 pM 1,25(OH)2D3, or 130 pM 1,25(OH)2D3 plus 6.5 nM 24,25(OH)2D3 for 5 minutes; determination of PKC activity revealed levels that were 170 ± 7, 340 ± 17, and 209 ± 17 pmol/minute/mg of protein, respectively. The decrease in PKC activity mediated by 24,25(OH)2D3 was found to be significant relative to enzyme activity in the presence of 1,25(OH)2D3 alone (P < 0.01). Parallel experiments in which cells were incubated for 7 minutes in the presence of 65 pM bPTH(3–34), 65 pM bPTH(1–34), or 65 pM bPTH(1–34) plus 6.5 nM 24,25(OH)2D3 exhibited PKA levels that were 11 ± 1.6, 25 ± 5, and 10 ± 1.5% of total cellular PKA activity, respectively (n = 3). The decrease in PKA activity mediated by 24,25(OH)2D3 was found to be significant relative to enzyme activity in the presence of bPTH(1–34) alone (P ≈ 0.05). Thus, 24,25(OH)2D3 attenuates the effects of both 1,25(OH)2D3 and PTH on activation of their respective protein kinases.
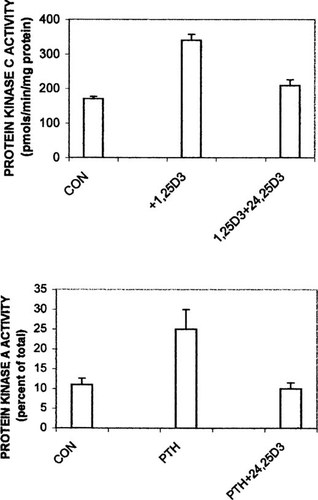
Inhibition of PKC and PKA activities by 24,25(OH)2D3. Isolated intestinal cells were incubated in microfuge tubes for 5 minutes (PKC) or 7 minutes (PKA) in the presence of control media (vehicle or bPTH(3–34)), 130 pM 1,25(OH)2D3, or 65 pM bPTH(1–34). Tubes were then chilled and cells pelleted by centrifugation. The cell pellets were homogenized and assayed for kinase activities as described in commercially available kits. Values represent mean ± SEM for three independent experiments.
DISCUSSION
The current work indicates that physiological levels of 24,25(OH)2D3 inhibit a wide range of 1,25(OH)2D3 concentrations that stimulate intestinal calcium transport in the perfused duodenal loop system. This finding may explain why it is difficult to observe an acute effect of 1,25(OH)2D3 on calcium absorption in vivo (I. Nemere, unpublished observations). In the perfused duodenal loop system, antagonism of the stimulatory effect of 1,25(OH)2D3 by 24,25(OH)2D3 was often seen 10 minutes after the onset of perfusion. A similar time lag was reported earlier for the 24,25(OH)2D3-mediated suppression of 1,25(OH)2D3-enhanced calcium uptake in osteoblasts.11
Although it could be argued that 24,25(OH)2D3 might be binding to and blocking the same receptor as 1,25(OH)2D3, a number of observations would indicate that the two metabolites act through separate receptors. As reported in 1994,20 the two metabolites do not compete well with each other for binding to basal lateral membrane preparations from chicks; and in addition, the binding moieties are selective for distinct analogs. Finally, preliminary characterization of the non-nuclear 24,25(OH)2D3 “receptor” suggests that it is compartmentalized differently than the non-nuclear receptor for 1,25(OH)2D3.21
24,25(OH)2D3 inhibits PTH-stimulated calcium transport as well, and it is highly unlikely that the seco-steroid and the peptide hormone act through the same receptors. Inhibition of the peptide hormone's action was surprising in view of an earlier report in which it was found that the stimulatory effect of PTH on phosphate transport in intestine was completely unaffected by 24,25(OH)2D3.15 These data suggest that a fundamental difference exists between activation of the transport pathways for calcium and phosphate.
In the present study, 1,25(OH)2D3 was found to stimulate rapid oscillations in ionized intracellular calcium, as judged by fura-2 fluorescence. The oscillatory pattern observed in microscopic fields encompassing several hundred cells might in part be attributed to the continued attachment of some of the isolated cells by means of junctional complexes; this in turn might facilitate communication between cells. 24,25(OH)2D3 also induced calcium oscillations in isolated intestinal cells, although with a qualitative difference; between “spikes” of fluorescence: Fura-2 emissions diminished below basal levels, whereas in the presence of 1,25(OH)2D3 alone fluorescence below basal levels was more rare. In the presence of both seco-steroid hormones, the 24,25(OH)2D3 pattern predominated. Whether this represents inhibition of signal transduction is unknown. However, Lieberherr22 has reported that 24,25(OH)2D3 affects intracellular calcium stores rather than calcium channels in mouse osteoblasts, while Li et al.23 have found that 24,25(OH)2D3 modulates L-type calcium channels in UMR-106 cells through modulation of PKC and PKA activities.
1,25(OH)2D3 stimulates PKC activity, as reported by many others (for review see, Nemere and Farach-Carson24), as well as PKA activity, in agreement with the observations of de Boland and Norman.25 Simultaneous exposure of intestinal cells to both seco-steroids resulted in a significant decrease in PKC activity (as well as PKA activity, data not shown), supporting the role of PKC in signal transduction of the rapid effects of these hormones.
PTH has been reported to directly affect calcium in isolated rat intestinal cells26, 27 and in perfused duodenal loops of chickens.28 The mRNA for the PTH receptor has been found in intestinal epithelium29 as has specific saturable binding to basal lateral membranes.15 The present observation that PTH activates PKA in isolated epithelial cells provides additional evidence that the peptide hormone acts directly on intestine. The finding that 24,25(OH)2D3 blocks PTH-stimulated signal transduction through the PKA cascade, suggests that the seco-steroid may be a major contributor to the regulation of calcium homeostasis in vivo.
The combined data indicate that 24,25(OH)2D3 exerts physiologically significant endocrine actions in chick intestine. In view of the evolutionary conservation of responsiveness to 24,25(OH)2D3 in multiple tissues of fish, chick, rat, and humans, the endocrine actions of this vitamin D metabolite are most likely of fundamental importance.
Acknowledgements
I.N. thanks Dr. Ken White for the use of his equipment to analyze fura-2 fluorescence and Ken Campbell for technical assistance. 1,25(OH)2D3 was the generous gift of Dr. Milan Uskokovic, Hoffmann-LaRoche, Nutely, NJ, 24,25(OH)2D3 was the generous gift of Kureha Chemical Co., Tokyo, Japan. These studies were funded by the National Research Initiative Competitive Grants Program/USDA 98-35200-6466, and by the Utah Agricultural Experiment Station, Utah State University, Logan, Utah, U.S.A. Approved as journal paper no. 7132.




