The Nucleolar Targeting Signal (NTS) of Parathyroid Hormone Related Protein Mediates Endocytosis and Nucleolar Translocation
Abstract
Previous work has identified the parathyroid hormone–related protein (PTHrP) nucleolar targeting signal (NTS) as both necessary and sufficient for localization of PTHrP to the nucleus and nucleolus of a variety of cells where it is believed to participate in the regulation of cell proliferation, differentiation, and apoptotic cell death. The mechanism whereby a secreted peptide, such as PTHrP, gains access to the nuclear compartment remains a question of debate. The current work examines the possibility that exogenous PTHrP is internalized and transported to the nuclear compartment by a mechanism that is dependent on preservation of the PTHrP NTS. Transiently expressed, PTHrP(1–141) was detected at the cell surface as well as in the cytoplasmic and nuclear compartments of COS-1 cells. Deletion of the NTS, or mutation of the conserved GxKKxxK motif within the NTS, effectively prevented both cell-surface binding and nuclear/nucleolar accumulation of PTHrP(1–141). A biotinylated peptide corresponding to the PTHrP NTS (PTHrP-NTS-biotin) was internalized and translocated to the nucleus and nucleolus in a time-, temperature-, and concentration-dependent manner, whereas a peptide representing a similar bipartite NTS from Nucleolin was not. Internalization and nucleolar targeting of PTHrP-NTS-biotin were indistinguishable in CFK2 cells, which express the common PTH/PTHrP receptor, and in 27m21 cells, which do not. In addition, pretreatment with a saturating dose of synthetic PTHrP(74–113) was capable of abrogating nucleolar accumulation of the PTHrP-NTS peptide, whereas pretreatment with PTHrP(1–34) or PTHrP(67–86) was not. These observations demonstrate that binding of exogenous, full-length PTHrP to the cell surface is mediated through a conserved motif embedded in the NTS and suggest that internalization and nucleolar targeting of an NTS peptide are mediated through binding to a cell surface protein distinct from the PTH/PTHrP receptor. In total, the data support the hypothesis that secreted PTHrP(1–141) can be endocytosed and targeted to the nucleolus through a mechanism that is dependent on preservation of a core motif within the PTHrP NTS.
INTRODUCTION
PARATHYROID HORMONE–RELATED PROTEIN (PTHrP) was discovered as a tumor-derived protein that is associated with the common paraneoplastic syndrome of humoral hypercalcemia of malignancy.1 Most cases of humoral hypercalcemia of malignancy are caused by high levels of circulating PTHrP that activate the classic parathyroid hormone (PTH) receptor in kidney and bone to elicit similar abnormalities in calcium and phosphate metabolism as seen in patients with hyperparathyroidism.2 These effects were shown to be a function of limited sequence homology at the amino termini of PTH and PTHrP which allows them to bind to and activate the same receptor, subsequently named the PTH/PTHrP receptor.3
A second cell surface receptor (PTH2R) has recently been cloned that shares only 20% sequence homology with the PTH/PTHrP receptor (PTH1R), has a much more limited distribution and binds PTH with much higher affinity than PTHrP.4 Evidence also exists for the tissue-specific expression of alternative transcripts and splice variants of rodent PTH1R.5, 6 In one of those variants the signal peptide is spliced out, resulting in a nonsecreted form of the receptor which has been localized to the cytoplasm when expressed in COS-1 cells.6 Restricted expression patterns of putative cell surface binding proteins for midregion species of PTHrP have also been documented.7
The rodent PTHrP gene encodes a single peptide of 141 amino acids that undergoes endoproteolytic cleavage in the secretory pathway.8 In addition to intact PTHrP(1–141), amino-terminal fragments of PTHrP(1–36) and PTHrP(1–86) are believed to be released into the extracellular environment, along with midregion PTHrP(38–94) and PTHrP(38–101), in a cell type–specific manner.9, 10 Most of the documented biological activity of PTHrP is believed to be mediated through the interaction of its amino terminus with the common PTH1R. However, additional evidence exists for bioactivity mediated by midregion and carboxy-terminal species, through as yet unidentified mechanisms.11-16 In this context, it has been shown that amino acids 87–107 of PTHrP constitute a nuclear/nucleolar targeting signal (NTS) that shares sequence similarity to both the lysine-rich bipartite sequences seen in proteins such as nucleolin, as well as to the arginine-rich NTS in Tat. In addition to its function as a NTS,17 the Tat NTS has been implicated in binding and endocytosis of exogenous Tat peptides,18, 19 in mediating interactions with the long terminal repeat in nascent viral RNA20, 21 and in regulating proliferation and apoptosis in cultured cells.22, 23 The PTHrP NTS is necessary and sufficient to direct the passage of both transfected PTHrP and β-galactosidase, a heterologous, cytoplasmic protein, to the nuclear compartment. PTHrP has been localized to the nucleolus of skeletal cells in vivo and has been implicated in the modification of cell cycle progression15 differentiation and apoptotic death of cells in vitro.13, 14 In addition, we have recently demonstrated that PTHrP binds to RNA through its NTS.24
In total, the preceding data suggest that: multiple fragments of PTHrP are secreted along with the full-length mature protein; multiple receptor subtypes for PTHrP are expressed both at the cell surface and in the cytoplasm; and the bioactivity of PTHrP associated with cell growth and differentiation might be mediated directly, in the cytoplasmic or nuclear compartments, rather than indirectly through classic signal transduction cascades. These observations have given rise to the hypothesis that secreted PTHrP re-enters the cell, through an endocytotic mechanism, to be transported to the nuclear compartment.
In the current work, we have demonstrated that full-length PTHrP(1–141) localizes to the plasma membrane and to the cytoplasmic, nuclear, and nucleolar compartments of transfected COS-1 cells; that cell surface attachment and nuclear/nucleolar localization are dependent on a conserved motif embedded in the NTS, and that a biotinylated NTS peptide localizes to the nucleus/nucleolus by an endocytotic mechanism in a time- and dose-dependent manner. Taken together, these observations suggest that secreted PTHrP has the potential to directly influence cellular function through mechanisms distinct from those mediated indirectly through classical signal transduction cascades linked to the PTH/PTHrP receptor.
MATERIALS AND METHODS
Plasmids and peptides
The plasmids PTHrPmyc, -NTSPTHrPmyc (same as Δ87–107PTHrPmyc), M1PTHrPmyc, M2PTHrPmyc, and M3PTHrPmyc, that are shown in Fig. 1A, have been described previously.24 Plasmid pEFc-myc, encoding full-length human c-myc was a kind gift of A. Koromilas (Lady Davis Institute, Montreal, Canada) and plasmid pCBP1677-1897myc, which encodes a myc-tagged fragment of CBP, was a kind gift of R. Lin.25
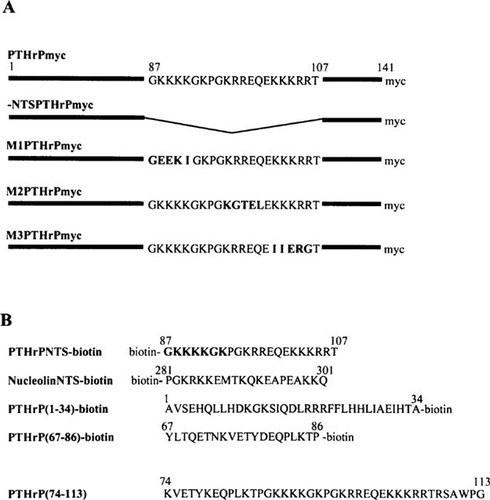
Plasmids and peptides used in the characterization of PTHrP endocytosis and nuclear translocation. (A) cDNA constructs encoding rat PTHrP, -NTSPTHrP, and NTS mutant M1, M2, and M3 forms of PTHrP that were modified to include an in-frame myc epitope tag for use in the COS-1 transfection assays. (B) The amino acid sequences of the various synthetic peptides that were biotinylated for use in the subcellular localization experiments performed in CFK2 and 27m21 chondrocytic cells.
Synthetic PTHrP(1–34) and PTHrP(67–86) were purchased from Peninsula Labs (Belmont, CA, U.S.A.). The peptides shown in Fig. 1B were all synthesized at the Sheldon Institute for Biotechnology (McGill University, Montreal, Canada). The Sheldon Center uses a SYMPHONY MutltiplexTM peptide synthesizer that allows automated, cyclical amino acid addition with minimal operator intervention. When complete, the peptides are cleaved from the resin, precipitated, and purified by reverse-phase high-performance liquid chromatorgaphy. Peptide sequence identity was confirmed using mass spectrometry (MALDI-TOF; Perceptive Biosystems, Perkin Elmer, Foster City, CA, U.S.A.).
Cell culture, transient transfection and protein harvest
All cell culture reagents were purchased from Life Technologies (Grand Island, NY, U.S.A.) and all plasticware (Falcon) from Becton Dickinson Labware (Lincoln Park, NJ, U.S.A.). CFK2 and 27m21 chondrocytic cells were maintained in RPMI 1640 and Dulbecco's modified Eagle's medium, respectively, supplemented with 10% fetal bovine serum and antimycotic-antibiotic. COS-1 cells were maintained and transfected as described previously, using mock-transfected and vector-transfected cells for control.13 For immunoblot analysis of whole cell lysates, transfected cells were harvested by scraping into lysis buffer (150 mM NaCl, 20 mM Tris pH 7.4, 20 mM NaF, 0.1 mM sodium vanadate, 1% Triton X-100, 500 μM phenylmethylsulfonyl fluoride, 20 μg/ml leupeptin, and 15 μg/ml aprotinin).
For immunoprecipitation analysis of proteins secreted into the culture medium, 106 cells were transfected in 100-mm dishes and maintained for 48 h post-transfection. Medium was then replaced with 3 ml of fresh medium supplemented with 20 μg/ml aprotinin and 15 μg/ml leupeptin. Phenylmethylsulfonyl fluoride was added to a final concentration of 50 μM. Conditioned medium was harvested after 24 h and kept frozen until the time of analysis.
Immunoblot and immunoprecipitation analysis
The polyclonal antiserum raised against rat PTHrP(1–34) has been previously characterized.26 The monoclonal antibody 9E10, specific for the human c-myc epitope,27 was harvested as ascites fluid from mice 7 days after implantation of the hybridoma (ATCC). Horseradish peroxidase–conjugated secondary antisera were purchased from Sigma (St. Louis, MO, U.S.A.). Immunoblot and immunoprecipitation analyses were performed as described.24 In brief, whole cell lysates, prepared from mock-transfected COS-1 cells and cells transfected with the various constructs, were denatured in Laemmli sample buffer. The proteins were separated by size on 15% SDS-polyacrylamide gels and transfered to nitrocellulose membranes. Membranes were blocked overnight with 5% nonfat milk protein in Tris-buffered saline with 0.05% Tween-20 and incubated overnight with 9E10 primary antibody. The protein bands were detected using horseradish peroxidase–conjugated secondary antibody and the enhanced chemiluminescence system (Amersham, Arlington Heights, IL, U.S.A.).
Conditioned medium harvested from COS-1 cells expressing wild-type and mutant PTHrP proteins was incubated with anti-PTHrP(1–34) at 4°C overnight and immunoprecipitated with protein-A Sepharose prior to denaturing in Laemmli buffer and performing immunoblot analysis, as described, using the 9E10 antibody.
Bioassay of wild-type and mutant proteins
Inhibition of sodium-dependent phosphate transport was used as an index of amino-terminal PTHrP bioactivity14, 24 in medium harvested from transfected COS-1 cells. Briefly, confluent layers of opossum kidney epithelial cells (OK/E) were stimulated for 3 h in serum-free medium with serial dilutions of synthetic PTHrP(1–34) or medium harvested from the transfected COS cells. Transport of [32P]orthophosphate was carried out for 5 minutes and terminated by addition of ice-cold wash buffer before counting the solubilized cell layers to assess32P incorporation.
Immunofluorescence microscopy
Fluorescein isothiocyanate (FITC)–conjugated anti-rabbit and tetramethyl-rhodamine-isothiocyanate (TRITC)–conjugated anti-mouse antisera were purchased from Sigma and Boehringer Mannheim (Mannheim, Germany, U.S.A.), respectively. Localization of PTHrP in transfected COS-1 cells was performed essentially as described previously.13 Briefly, cells on glass cover-slips were fixed in 4% paraformaldehyde to preserve surface antigens prior to fixation with methanol/acetone. Colocalization of the amino and carboxyl termini of PTHrP was accomplished by staining concurrently with rabbit anti-PTHrP(1–34) and mouse 9E10 antibody (carboxyl terminus). FITC-conjugated (yellow) anti-rabbit and TRITC-conjugated (red) anti-mouse secondary antibodies were used for detection of the amino and carboxyl termini, respectively. Full-length c-myc was expressed as a localization control for staining with 9E10 antibody that recognizes the 11 amino acid myc epitope tag. All cells within a field were identified by poststaining with Hoechst 33258 (Sigma) to identify nuclei.
For the quantitation of nucleolar staining, a total of 200 transfected cells on two different coverslips/transfection were scored and the number was expressed as a percentage of the total number of transfectants counted.
Localization of biotin-conjugated NTS peptides
CFK2 cells were plated at a density of 3 × 104 cells/well in 24-well plates on glass coverslips 24 h prior to treatment under the specified conditions. Peptides were diluted to the indicated concentrations in RPMI containing 0.1% BSA and used to treat CFK2 and 27m21 cells for the specified lengths of time. Treated cells were washed twice with PBS, fixed for 10 minutes with 4% paraformaldehyde, permeabilized for 10 minutes with ice-cold methanol, and blocked for 30 minutes with 10% BSA. The cells were then incubated for 45 minutes at room temperature with 40 μg/ml streptavidin-FITC (Boehringer Mannheim) in PBST containing 2% BSA. After rinsing, coverslips were mounted in 75% glycerol and photographed as described.13 Competition experiments, to saturate binding sites, were performed by pretreating cultures for 1 h with competing peptides at a concentration of 10−7 M prior to incubating with NTS-biotin for 90 minutes.
RESULTS
In previous work, it was shown that PTHrP localized to the nucleus and nucleolus of a subpopulation of transiently transfected COS-1 cells. Nuclear/nucleolar localization of PTHrP was dependent on the presence of an NTS, located between amino acids 87–107 of the protein, which was also shown to be both necessary and sufficient to target a heterologous cytoplasmic protein to the nuclear compartment. This work did not address the question of the species of PTHrP that entered the nuclear compartment or how it got there. Based on the hypothesis that secreted, full-length PTHrP reaches the nucleus following endocytosis, the objectives of the current work were 3-fold: to determine if intact PTHrP(1–141) localized to the cell surface and nucleolus of transfected cells; to assess the effect of conservative mutations within the NTS on cell surface binding and nuclear targeting of PTHrP; and to determine if an exogenous peptide, corresponding to the NTS, could enter the cell and localize to the nucleus and nucleolus.
Subcellular localization of wild-type and mutant PTHrP proteins in COS-1 cells
Figure 1A depicts the conservative mutations generated within the PTHrP NTS and Fig. 1B shows the synthetic peptides used in these studies. The antisera used previously for immunolocalization of PTHrP were raised against synthetic PTHrP(1–34) and PTHrP(67–86). Neither of these antisera provided information regarding the presence of the carboxy-terminal domain of the protein, for which specific and sensitive antisera have yet to be developed. To facilitate identification of the expressed proteins, and permit colocalization of amino and carboxyl termini, we generated constructs encoding wild-type and mutant forms of PTHrP with a myc epitope tag at the carboxyl terminus. We have used triple-label immunofluorescence microscopy to colocalize the amino and carboxyl termini of PTHrP using FITC-conjugated (yellow) anti-rabbit and TRITC-conjugated (red) anti-mouse secondary antisera, respectively, and Hoechst 33258 (blue) nuclear stain. The fixation protocol was modified to preserve cell surface antigens. Both amino (Fig. 2A) and carboxyl (Fig. 2C) termini of wild-type PTHrPmyc were localized to the Golgi, cytoplasmic and nuclear compartments of transfected COS-1 cells. Using Hoechst 33258 (Fig. 2B) to localize the nuclei of all cells, staining was apparent at the surface of both transfected cells (Figs. 2A and 2C, arrows) and their untransfected neighbors (Figs. 2A and 2C, arrowheads). Figures 2D and 2E show exclusive nuclear localization of full-length c-myc indicating that the nucleolar, cytoplasmic, and cell surface localization of the PTHrPmyc fusion protein was not a staining artifact. Expression of a cDNA encoding the myc epitope tag alone resulted in background staining. Expression of -NTSPTHrPmyc (Figs. 2F and 2G) resulted in staining that was restricted to a punctate pattern in the cytoplasm and intense staining in the Golgi apparatus. However, the cell surface, nucleus, and nucleolus were devoid of staining.
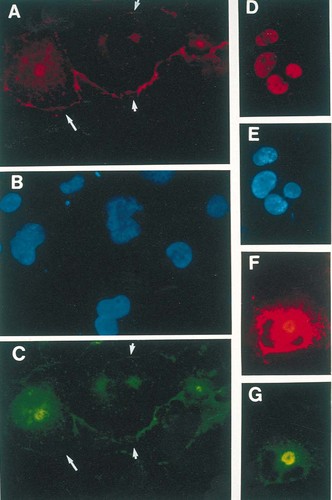
Subcellular localization of wild-type and mutant PTHrP proteins in COS-1 cells. In cells fixed and stained 48 h after transfection, colocalization of amino (A) and carboxyl (C) termini of wild-type PTHrP (PTHrPmyc) was observed at the cell membrane (arrows), in the Golgi, cytoplasm, and nucleus of transiently transfected COS-1 cells. Cell surface staining was also seen at the membrane (arrowheads) of untransfected cells, identified using Hoechst 33258 nuclear stain (B). The staining for c-myc (D) was restricted to the nucleus (E). Conversely, expression of -NTSPTHrPmyc, which is devoid of the NTS, resulted in staining that was restricted to the Golgi network and the cytoplasm (F, G). The results are representative of more than five different transfections.
Although deletion of the NTS resulted in abrogation of nucleolar staining, preliminary work demonstrating selective substitutions within this domain had variable effects on targeting of the protein to the nucleus and nucleolus.28 Mutation of87GKKKK91 to87GEEKI91 (M1) was almost as effective as deletion of the NTS in blocking nuclear targeting of PTHrP. In contrast, substitution of102KKKRR106 for102IIERG106 (M3) was only partially effective in this respect and mutation of96KRREQ100 to96KGTEL100 (M2) had no effect. Figure 3 shows the percentage of randomly cycling transfected COS-1 cells in which nucleolar accumulation of the epitope-tagged proteins was evident. In keeping with our previous results, deletion of the NTS abolished nucleolar accumulation of PTHrP, which was evident in ∼19% of transfected cells and in ∼20% of M2PTHrPmyc expressing cells. In contrast, nucleolar accumulation of M1PTHrPmyc was reduced to ∼7% and that of M3PTHrPmyc to ∼13% of transfected cells.

Quantitation of nuclear staining in COS-1 cells expressing wild-type and mutant forms of PTHrP. Populations of cells transiently transfected with the wild-type and mutant PTHrP cDNAs were scored for the presence of nucleolar PTHrP. The mean ± SD were plotted as a percentage of 200 transfected cells/cover slip in three different experiments.
These observations indicated that the appearance of full-length PTHrP at the surface and in the nucleus/nucleolus of COS-1 cells was dependent on the presence of an intact NTS and that nucleolar translocation was dependent on the preservation of amino acids 87–91 at the amino terminus of the NTS.
Immunochemical analysis of wild-type and mutant forms of PTHrP in transfected COS-1 cells
To confirm that the observations were not a consequence of variable levels of protein expression, lysates of COS-1 cells expressing the myc-tagged proteins were subjected to immunoblot analysis using the 9E10 anti-myc antibody. (Fig. 4A). All of the proteins were expressed at approximately equivalent levels and did not undergo significant intracellular processing. A major band corresponding in size to 25–27 kDa was observed for PTHrPmyc and the three mutant proteins, M1, M2, and M3PTHrPmyc. A faster migrating band corresponding to 22–24 kDa was observed for -NTSPTHrPmyc, in keeping with the 20 amino acid deletion. Mock transfected cells and cells expressing an unrelated protein carrying a myc epitope tag were used as negative and positive (for 9E10) controls, resepectively. Figure 4B shows a Coomasie blue stained gel, run in parallel, to verify equivalent loading of the lanes.
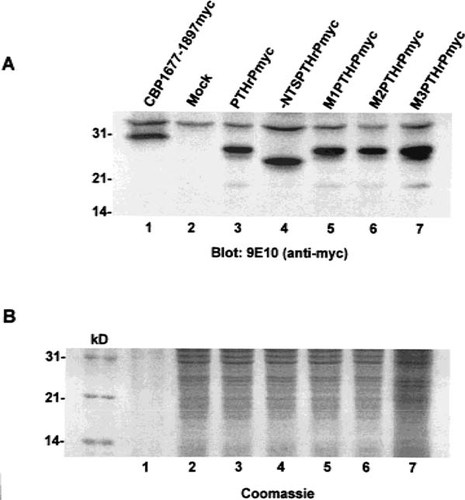
Immunochemical analysis of wild-type and mutant forms of PTHrP in transfected COS-1 cells. Whole cell lysates were prepared 36 h after transfection from COS-1 cells expressing wild-type and mutant proteins and subjected to immunoblot analysis using 9E10 antimyc antibody (A). Lane 1 shows expression of an unrelated protein, of comparable size, fused in frame with the myc epitope tag, and lane 2 shows a mock-transfected lysate as a negative control. A major band corresponding in size to 25–27 kDa was observed for PTHrPmyc (lane 3) M1 (lane 5), M2 (lane 6), and M3PTHrPmyc (lane 7). A faster migrating band corresponding to 22–24 kDa was observed for -NTSPTHrPmyc (lane 4), in keeping with the 20 amino acid deletion. (B) shows the corresponding Coomasie blue stained gel, run in parallel, to verify equivalent loading of the lanes. kD = molecular size marker. The results are representative of four separate analyses using three different lysates.
Secretion and amino-terminal bioactivity of the proteins expressed by COS-1 cells
We next wished to verify that the wild-type and mutant proteins were secreted intact and in a biologically active state from the transfected COS-1 cells. Conditioned medium harvested from the transfected cells was subjected to immunoprecipitation and immunoblot analysis and assessed for amino-terminal bioactivity in the OK/E phosphate transport assay.14 Proteins immunoprecipitated from conditioned medium with the anti-PTHrP (amino-terminal) antiserum and detected with the anti-myc antibody (carboxy-terminal) were present in approximately equivalent quantities and corresponded in size to those identified in the cell lysates (Fig. 5A). This indicated that there were no significant differences in processing and/or degradation as a consequence of deletion or mutation of the NTS sequence. As shown in Fig. 5B, the culture media containing the secreted peptides were also equipotent in their capacity to inhibit the transport of radiolabeled phosphate into OK cells in a dose-dependent manner. When diluted 1:10 with unconditioned medium their bioactivity was comparable to that elicited by 5 × 10−10 M synthetic bPTH(1–34) (Fig. 5B, lower right).
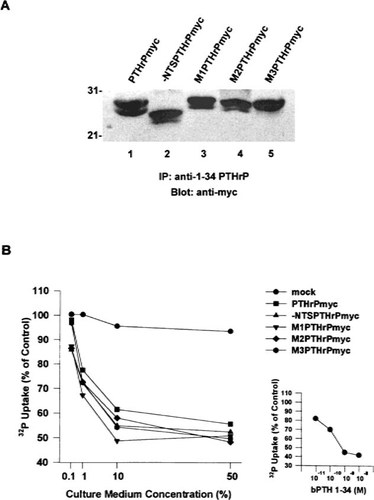
Secretion and amino-terminal bioactivity of the proteins expressed by COS-1 cells. Proteins were immunoprecipitated (IP) with anti-PTHrP(1–34) antiserum from medium collected over a 24-h period at 48 h post-transfection. IPs were resolved on 15% SDS-PAGE gels and transfered to nitrocelluose membranes which were probed with the 9E10 anti-myc antibody (A). Species incorporating both amino (IP) and carboxyl (immunoblot) termini were present in approximately equivalent quantities and corresponded in size to those identified in the cell lysates. Amino-terminal bioactivity of serial dilutions of conditioned medium containing wild-type and mutant proteins, as assessed in the OK/E phosphate transport assay, was approximately equivalent (B) and equipotent at a 1:10 dilution with 5 × 10−10 synthetic bPTH(1–34) (lower right). The results are expressed as a percentage of baseline activity which was 5717 ± 295 cpm of incorporated32P and are representative of three separate assays. Mock = mock transfection.
In total, the observations using transiently over-expressed proteins indicated that: deletion of the NTS from PTHrP resulted in the loss of cell surface binding and nuclear/nucleolar accumulation; wild-type and mutant PTHrP carrying the carboxy-terminal myc epitope tag were expressed and secreted equally well by COS-1 cells; and mutation within a conserved motif embedded in the NTS severely compromised cell surface attachment (data not shown) and nucleolar targeting. Subsequent experiments were focussed on defining the role played by the PTHrP NTS in mediating internalization and nuclear targeting.
Time dependence of nucleolar translocation of exogenous PTHrP-NTS peptide
Given our observations that cell surface attachment and nucleolar targeting of wild-type PTHrP(1–141) was mediated through the NTS we next tested the hypothesis that exogenous PTHrP-NTS peptide could be internalized and translocated to the nucleolus. For these studies we used the CFK2 chondrocytic cell line which expresses the PTH/PTHrP receptor and has been shown to be a physiological target for PTHrP.13, 14 To assess the role played by the PTH/PTHrP receptor in endocytosis and nuclear targeting of PTHrP we used a comparable chondrocytic line, 27m21 (which does not express the PTH/PTHrP receptor). Biotinylated peptides were localized using streptavidin-fluorescein.
Subconfluent CFK2 cells treated with PTHrP-NTS-biotin at 37°C were harvested at timed intervals and assessed for staining using FITC-conjugated streptavidin (Fig. 6). Diffuse cytoplasmic staining was observed at the periphery of cells harvested at 15 minutes (Fig. 6A, arrowheads), whereas nucleolar accumulation of PTHrP-NTS-biotin was first evident at 30 minutes (Fig. 6B, arrows) and further increased by 90 minutes (Fig. 6C) of treatment. There was no further increase in nuclear/nucleolar accumulation for treatment periods in excess of 90 minutes, merely an increase in nonspecific accumulation of the peptide in the intercellular spaces (data not shown). When the cells were treated with the same peptide concentration at 4°C (lower panels) there was no diffuse cytoplasmic staining or nucleolar accumulation of the PTHrP-NTS-biotin peptide (Figs. 6D–6F). The nonspecific punctate, cytoplasmic staining evident at all time points, under all conditions, is most probably a function of streptavidin binding to endogenous biotin-containing proteins.29
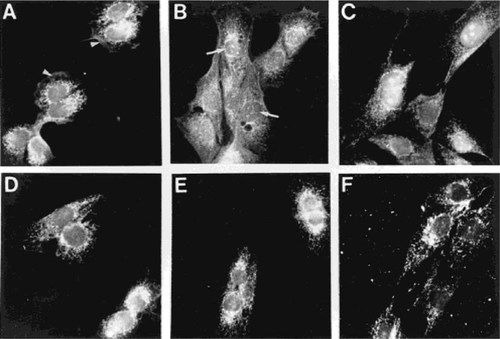
Time dependence of nucleolar translocation of exogenous PTHrP-NTS peptide. CFK2 cells were treated with 10−7 M biotinylated peptide corresponding to amino acids 87–107 of PTHrP for timed intervals prior to fixation and localization of the biotin-tagged peptide with streptavidin-FITC (SF). While diffuse staining is present at the cell membrane and in the cytoplasm at 15 minutes (A, arrowheads), nuclear and nucleolar accumulation is first evident at 30 minutes (B, arrows) and increased at 90 minutes (C) of incubation. Cells treated for equivalent periods of time at 4°C, with the same peptide concentration, do not exhibit specific staining at any time point (D–F). The results are representative of four different assays.
Dose dependence and specificity of internalization of PTHrP-NTS peptide
The observations of time and temperature dependence of nucleolar accumulation suggested the presence of a specific, saturable endocytotic mechanism for the NTS peptide.30 Additional support for this hypothesis came from experiments in which the cells were treated with increasing concentrations of NTS-biotin peptide (Figs. 7A–7C). A minimum concentration of 10−7 M (Fig. 7B) was required to detect distinct nucleolar accumulation of the peptide at 90 minutes, with an increase in nuclear and nucleolar staining observed at 10−5 M peptide (Fig. 7C).
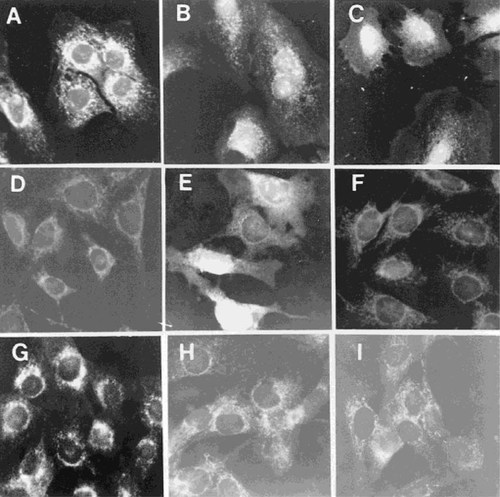
Dose dependence and specificity of internalization of PTHrP-NTS peptide. A dose-dependent increase in nucleolar accumulation of PTHrP-NTS-biotin is evident in CFK2 cells treated at 37°C with 10−9 M (A), 10−7 M (B) or 10−5 M (C) PTHrP-NTS-biotin. 27m21 chondrocytic cells, which do not express the PTH/PTHrP receptor, also showed nucleolar accumulation of peptide when treated with 10−7 M PTHrP-NTS-biotin for 90 minutes (E). 27m21 cells treated with strepatavidin-fluorescein alone (D) or with a 10-fold excess of unconjugated PTHrP-NTS (F) failed to demonstrate nuclear or nucleolar staining. Similarly, cells exposed to 10−7 M NucleolinNTS-biotin (G), PTHrP(1–34)-biotin (H) or PTHrP(67–86)-biotin (I) failed to show any specific staining. The results are representative of three to six separate experiments.
To determine if endocytosis and translocation of the PTHrP-NTS-biotin peptide was related to the presence of the PTH/PTHrP receptor we incubated 27m21 cells, which have no PTH/PTHrP receptor, with streptavidin-fluorescein alone (Fig. 7D), with 10−7 M PTHrP-NTS-biotin (Fig. 7E) or with 10−7 M PTHrP-NTS-biotin in the presence of 10× excess nonbiotinylated PTHrP-NTS (Fig. 7F). It was evident that the staining pattern, in the absence of the PTH/PTHrP receptor, was identical to that seen in the receptor-expressing CFK2 cells (compare Figs. 7B and 7E).
The specificity of the nuclear/nucleolar staining was further demonstrated by incubating CFK2 with biotinylated peptides corresponding to NucleolinNTS (Fig. 7G), to PTHrP(1–34) (Fig. 7H) and to PTHrP(67–86) (Fig. 7I). None of these peptides demonstrate specific nuclear or nucleolar staining. Additionally, CFK2 cells treated with unconjugated biotin, strepatavidin-fluorescein alone or coincubated with PTHrP-NTS-biotin and a 10-fold excess of unconjugated PTHrP-NTS peptide showed no specific staining (data not shown).
The fact that the nucleolin peptide, which is very basic and shares considerable sequence homology with the PTHrP NTS (see Fig. 1), was not internalized suggested that cell-surface binding was not a random event but was mediated through recognition of the PTHrP NTS per se. To confirm this hypothesis, the CFK2 cells were pretreated with saturating doses of synthetic PTHrP(1–34) or PTHrP(74–113) to block the binding sites for amino and midregion species of PTHrP, respectively. A 1 h pretreatment with 10−7 M PTHrP(74–113) was effective in completely blocking the entry of NTS-biotin into CFK2 cells over a 90-minute incubation period (Fig. 8B) compared with cells receiving no pretreatment (Fig. 8A). An equivalent concentration of PTHrP(1–34) was ineffective in this respect (Fig. 8C). These experiments provided further support for our hypothesis that endocytosis and nuclear/nucleolar targeting of PTHrP were mediated through the NTS and did not involve the PTH/PTHrP receptor.

Effect of competition binding with PTHrP fragments on NTS peptide internalization. Binding sites for amino-terminal and midregion species of PTHrP were saturated by treating the CFK2 cells for 1 h with 10−7 M PTHrP(1–34) or with 10−7 M synthetic PTHrP(74–113). Cultures were then incubated with PTHrP-NTS-biotin for 90 minutes and the internalized peptide located with streptavidin fluorescein. Pretreatment with PTHrP(74–113) completely abrogated entry of PTHrP-NTS-biotin into CFK2 cells over a 90-minute incubation period (B) compared with cells receiving no pretreatment (A) or those pretreated with PTHrP(1–34) at an equivalent concentration (C). The results are representative of three different assays.
DISCUSSION
Taken together, our observations using chondrocytic cells treated with exogenous, biotin-tagged peptides demonstrated that internalization and nucleolar accumulation of the PTHrP-NTS-biotin peptide was specific, saturable, and mediated through an endocytotic mechanism that was not directly related to the presence of the PTH/PTHrP amino-terminal receptor.
Traditionally, regulation of cellular function by extracellular peptide growth factors and cytokines has been viewed as an indirect process mediated through binding to a high-affinity receptor and activation of conventional signal transduction cascades. It is now evident that a variety of these factors, including epidermal growth factor, platelet-derived growth factor, fibroblast growth factors (FGFs), angiogenin, interleukins, and PTHrP also regulate cellular activity through direct association with the nuclei of target cells.31, 32 Despite their small size, nuclear localization of these peptides has been shown to be dependent on the presence of one or more specific targeting motifs termed nuclear localization sequences or NTSs. Since these proteins are considerably smaller than 45 kDa they should not require facilitated (nuclear localization sequence–mediated) transport into the nuclear compartment. It has therefore been suggested that these ligands might mediate the cotransport of larger molecules, including their cognate receptors, into the nucleus. Indeed this hypothesis is supported by observations from different investigators who have reported nuclear accumulation of interleukins,33, 34 FGFs,35, 36 angiogenin,37 and the retroviral regulatory protein Tat19, 21 following receptor- mediated endocytosis.
Although the pathways whereby extracellular ligands reach the nuclear compartment remains controversial it is apparent that more than one mechanism is involved. For instance, epidermal growth factor and FGF are believed to be transported to the nucleus in association with their high-affinity receptors.36, 38, 39 There is no direct evidence to date for nuclear translocation of G protein–coupled receptors. However, recent documentation of tissue-specific and alternatively spliced isoforms of the PTH/PTHrP receptor, including an intracellular species, raises the intriguing possibility that PTHrP could also translocate to the nucleus in association with a high-affinity receptor.5, 6 However, our current work suggests that, in chondrocytic cells, the PTH/PTHrP receptor is not required for nuclear translocation of PTHrP. Our observations are consistent with those of others who suggested internalization and nuclear translocation of FGF,40 angiogenin,37 and HIV-1 Tat19 occurred following interaction of NTS sequences with low-affinity, high-capacity, surface-binding proteins such as heparan sulfate proteoglycans. In the present work, we demonstrated that PTHrP bound to the surface of transfected cells that do not express the high-affinity PTH/PTHrP receptor and that binding was dependent on the presence of an intact NTS.
Whereas all transfected cells, as well as some of the neighboring untransfected cells, demonstrated cell surface binding of full-length PTHrP, nuclear/nucleolar accumulation of the intact protein was seen in only 15–20% of transfected cells. This most probably reflects the requirement for nuclear PTHrP at a specific stage of the cell cycle as has been suggested previously.13, 15, 16, 41 Nuclear accumulation of PTHrP42, 43 and FGF44 have also been proposed to be a function of alternative initiation of translation, to generate an NTS in FGF and to delete the signal peptide in PTHrP. In the presence of both a signal peptide and an NTS there would be a competition between opposing directional signals such that the signal sequence would direct the protein into the secretory pathway while the NTS would translocate it to the nuclear compartment.45 Our current studies with PTHrP demonstrated that, in a randomly cycling population of transfected cells, most had membrane-associated PTHrP, whereas only a small percentage demonstrated nuclear/nucleolar staining. These observations are consistent with the hypothesis that the PTHrP signal peptide takes precedence in directing the protein to the secretory pathway. However, under specific circumstances, PTHrP will either be endocytosed or undergo alternative translation and be translocated from the cytoplasmic to the nuclear compartment via a chaperone protein.
Recombinant Tat protein binds to a variety of target cells through a low-affinity, high-capacity binding protein.18, 19 Binding was shown to mediate entry of the peptide into unpermeabilized cells in a dose-, time-, and temperature-dependent manner. Subsequent studies using synthetic Tat peptides localized the region of interaction to amino acids 49–57 which represents the NTS and shares significant sequence homology with the PTHrP NTS.13 Similarly, extracellular peptides corresponding to the angiogenin NTS were internalized and translocated to the nucleolus of endothelial cells in culture.37 In the case of angiogenin and PTHrP, the NTS was also shown to be both necessary and sufficient to target heterologous proteins to the nuclear compartment. These observations suggested that the NTS alone mediated binding and endocytosis by physiologically relevant target cells. To address the specific role played by the PTHrP NTS in internalization and nucleolar targeting we employed two different chondrocytic cell lines. CFK2 cells express the high-affinity PTH/PTHrP receptor and demonstrate altered biological activity in response to nucleolar PTHrP.13, 14 27m21 cells are phenotypically similar to CFK2 but lack the PTH/PTHrP receptor. Like the Tat and angiogenin peptides, biotin-tagged PTHrP NTS peptide localized to the nucleus and nucleolus in a time-, concentration-, and temperature-dependent manner in both CFK2 and 27m21 cells. Conversely, a similar peptide that represents the bipartite nucleolar targeting sequence of nucleolin, a major nuclear-cytoplasmic shuttle protein, was not internalized or translocated to the nucleolus in CFK2 cells. Like NucleolinNTS-biotin, biotinylated amino-terminal, and midregion species of PTHrP lacking the NTS did not accumulate in the nucleolus. The fact that endocytosis and nuclear localization occurred in the absence of the amino terminus of PTHrP, and in cells that do not express the PTH/PTHrP receptor, indicates that these functions were not mediated through the high-affinity PTH/PTHrP receptor. This hypothesis was supported by the data showing that internalization and nucleolar targeting were competed out by cotreatment of the cells with peptides containing the NTS sequence.
It is important to note that while PTHrP(1–36) is most probably secreted as a natural cleavage product of PTHrP(1–141),9, 46 the nature of carboxy-terminal fragments is less well defined. Thus, PTHrP(38–141) may undergo further processing to be released as fragments of 38–95 or 38–101.10, 46 In both cases, these peptides incorporate the residues between amino acids 87–91, which we have shown, through mutagenesis, to be critical to cell surface binding (data not shown), nuclear/nucleolar translocation and RNA binding.24 One can then envisage a scenario whereby differential signaling could be accomplished by peptides secreted with an intact amino terminus and/or a functional NTS. In the former case, signal transduction would be elicited through the high-affinity G protein–linked receptor. In the later instance, PTHrP would mediate its effect directly in the nucleus following internalization. Alternatively, in the case of full-length PTHrP(1–141) simultaneous binding to high-and low-affinity receptors could occur.
In total this work demonstrates that full-length PTHrP, expressed from a rat cDNA encoding PTHrP(1–141), localizes to the plasma membrane, cytoplasmic, and nuclear compartments of COS-1 cells that do not express the high-affinity PTH/PTHrP receptor. Both membrane binding and nuclear translocation were dependent on preservation of a conserved GxKKxxK motif between amino acids 87–91, which also mediates RNA binding, and is involved in the protection of serum-deprived chondrocytes from apoptosis. In addition, a biotinylated peptide corresponding to the PTHrP-NTS was endocytosed and translocated to the nucleus of chondrocytes, in the presence and in the absence of the PTH/PTHrP receptor. On the basis of these observations, we suggest that carboxy-terminal fragments of PTHrP that lack an intact amino terminus have the potential to influence cellular function directly following internalization and translocation to the nuclear compartment.
Acknowledgements
We thank A.C. Karaplis for use of the PTHrP cDNAs used to generate the myc fusion constructs. We also thank B. Damaj for helpful discussion and B. He and S. Stregger for expert technical assistance. This work was supported by Grants to J.E.H. from the Medical Research Council of Canada (MRCC) and from the Fonds de la recherche en santé du Québec (FRSQ). J.E.H. is a Chercheur boursier of the FRSQ and M.M.A and A.R. were supported by studentships from the MRCC and Manpower Canada, respectively.




