A Chimeric Form of Osteoprotegerin Inhibits Hypercalcemia and Bone Resorption Induced by IL-1β, TNF-α, PTH, PTHrP, and 1,25(OH)2D3
Abstract
Osteoprotegerin (OPG) is a secreted protein that inhibits osteoclast formation and activity and appears to be a critical regulator of bone mass and metabolism. In the current study, mice were challenged with various cytokines and hormones (interleukin-1β, tumor necrosis factor-α, parathyroid hormone, parathyroid hormone-related protein, and 1α,25-dihydroxyvitamin D3) that are known to increase bone resorption and cause hypercalcemia and treated concurrently with either a recombinant chimeric Fc fusion form of human OPG, with enhanced biological activity (cOPG) (2.5 mg/kg/day) or vehicle. Mice receiving these bone-resorbing factors became hypercalcemic by day 3 after commencing treatment and had increased bone resorption as evidenced by elevated osteoclast numbers on day 5. Concurrent cOPG treatment prevented hypercalcemia (p < 0.05) and maintained osteoclast numbers in the normal range (p < 0.001). The demonstration that cOPG can inhibit bone resorption suggests that this molecule may be useful in the treatment of diseases including hyperparathyroidism, humoral hypercalcemia of malignancy, osteoporosis, and inflammatory bone disease, which are characterized, in part, by increases in osteoclastic bone resorption.
INTRODUCTION
OSTEOPROTEGERIN (OPG) is a novel secreted protein that has been shown to increase bone density in growing mice by inhibiting osteoclast differentiation and activation.1, 2 Moreover, transgenic mice over-expressing OPG were severely osteopetrotic,1 while mice deficient in OPG through targeted gene disruption exhibited early onset osteoporosis.3 These genetically altered animals indicate the importance of this new protein in the development and maintenance of normal bone mass and a role for OPG in inhibiting growth related bone resorption. OPG appears to act by binding a novel tumor necrosis factor (TNF) family member, OPG ligand (OPGL)4 (also known as TRANCE,5 RANKL,6 or ODF).7 OPGL is able to mediate osteoclast formation from mouse marrow cells in the absence of stromal cells and has been proposed to be the factor produced by osteoblasts to mediate the activity of hormones and cytokines on bone resorption.4, 7 If this is so, OPG should have broad antiresorptive activity against both cytokines and hormones implicated in metabolic and inflammatory bone disease.
The hypocalcemic effect of OPG has been recently shown in thyroparathyroidectomized rats.8 However, a specific effect on bone resorption is not reported. In this in vivo study, we have evaluated the ability of a chimeric Fc fusion form of OPG (cOPG) to inhibit the bone resorptive and calcemic effects of parathyroid hormone (PTH),9, 10 parathyroid hormone-related peptide (PTHrP),11, 12 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3),13, 14 TNF-α,15, 16 and interleukin (IL)-1β.17, 18 In addition, we have evaluated the action of cOPG on calcium levels and on the bone resorption induced in mice maintained on a low calcium diet.19
MATERIALS AND METHODS
Study protocol
Male BDF1 mice (Charles River Laboratories, Wilmington, MA, U.S.A.), n = 5, aged 5 weeks were injected daily for 4 days with either cytokine or hormone, and calvarial bone morphology was examined 24 h after the last injection. IL-1β (5 μg/day), TNF-α (50 μg/day), PTH (100 μg/day), PTHrP (20 μg/day), or vehicle alone (phosphate-buffered saline [PBS]) were injected into the subcutaneous tissue by the method described by Boyce et al.20 over the left side of the calvaria twice daily in a volume of 10 μl. This delivery method produces an exaggerated resorptive response in the calvarial bone as well as a systemic effect as indicated by hypercalcemia.20 1α,25(OH)2D3 (0.5 μg/day) or vehicle alone (corn oil) was injected subcutaneously once daily on the nape of the neck in a volume of 50 μl into animals on a low calcium diet, 0.02% (#5855 Low Calcium Diet; PMI Feeds, Inc., Richmond, IN, U.S.A.) versus 0.60% in the normal rodent diet (#5755 Basal Rodent Diet; PMI Feeds, Inc.). All animals were additionally treated with a recombinant chimeric construct of human OPG (cOPG) (2.5 mg/kg/day) or PBS vehicle, injected subcutaneously once daily on the flank. cOPG is a molecule comprising amino acids 22–194 of human OPG1 fused at the N terminus to the C terminus of the Fc domain of human immunoglobulin G1, and is covalently dimerized through the Fc domain.
Test materials, cOPG, IL-1β, and TNF-α were produced in E. coli by Amgen Inc. (Thousand Oaks, CA, U.S.A.) with recombinant methods. Human PTH (hPTH)(1–34), 1,25(OH)2D3, and research grade corn oil were purchased from the Sigma Chemical Co. (St. Louis, MO, U.S.A.), and hPTHrP(1–34) was purchased from Bachem Bioscience, Inc. (Torrance, PA, U.S.A.). Animals were housed in an AAALAC accredited facility and these studies were approved by the Lab Animal Research Committee of Amgen, Inc.
Whole blood ionized calcium measurement
Blood was collected retro-orbitally into heparinized capillary tubes and whole blood ionized calcium levels were determined with a Ca2+/pH analyzer (model 634; Chiron Diagnostics, Norwood, MA, U.S.A.) on days 0, 3, and 5, values were corrected to pH 7.4. Samples were taken prior to initiation of treatment to determine baseline ionized calcium levels, 3 h post-treatment on day 3 to determine the level of hypercalcemia, and at the time of sacrifice on day 5 (12 h after the last treatment). Animal weights were recorded at the same time points.
Histomorphometry and radiography
On day 5, after final ionized calcium levels were determined, the calvariae were removed, radiographed, and placed into buffered zinc formalin. Radiographs were taken with a Faxitron X-ray system (model 43855 A; Faxitron X-Ray Corp., Buffalo Grove, IL, U.S.A.). Calvaria were decalcified in formic acid, embedded in paraffin, and sectioned anterior and adjacent to the lambdoid suture. Sections were reacted to demonstrate tartrate-resistant acid phosphatase activity (TRAP) and counterstained with hematoxylin.
To determine the number of osteoclasts present in the bone, histomorphometic analysis was performed on the left side of the calvaria in the region of the injections (anterior and adjacent to the lambdoid suture). The area measured was a 3.0 mm × 0.5 mm rectangular area consisting of six 0.5 mm × 0.5 mm fields extending from lateral muscle attachment toward the midline suture and included all of the bone and marrow spaces. Only TRAP-positive osteoclasts found in contact with the endosteal surface were included in cell counts, with the exception of the PTHrP-treated group. The PTHrP-treated calvaria showed a distinct dorsal periosteal pattern of resorption, and therefore periosteal measurements were pooled with the endosteal measurements. Results were recorded as osteoclast number per unit bone tissue area measured (Oc.N/TAr). All measurements were made by tracing the section image onto a digitizing platen with the aid of a camera lucida attachment on the microscope and “Osteomeasure” (Osteometrics, Inc., Decatur, GA, U.S.A.) bone analysis software.
Serum PTH measurement
In an additional study, designed to determine the effects of cOPG treatment on calcium homeostasis, male BDF1 mice (Charles River Laboratories), n = 5, aged 5 weeks, were placed on either a low calcium diet, 0.02% calcium (#5855 Low Calcium Diet; PMI Feeds, Inc.) or normal rodent diet, 0.60% calcium (#5755 Basal Rodent Diet; PMI Feeds Inc.) and treated subcutaneously with cOPG (2.5 mg/kg/day) or vehicle alone (PBS) for 5 days. On day 5, blood was collected by cardiac puncture, and serum PTH levels were determined by immunoradiometric assay (Nichols Diagnostics, Inc., San Juan Capistriano, CA, U.S.A.).
Statistical analysis
For all analyses, means ± SEM are reported. The statistical significance of differences between means of treatment groups and the vehicle-treated controls were evaluated by Dunnett's test to allow for multiple comparisons.21 The statistical significance of effects of concurrent cOPG administration on group means for each bone resorption stimulator were evaluated by a Student's t-test.
RESULTS
Whole blood ionized calcium levels
Whole blood ionized calcium levels were elevated above control by day 3, 3 h postinjection of cytokine or hormone (Fig. 1). Treatment with each of IL-1β, TNF-α, PTH, PTHrP, or 1,25(OH)2D3 elevated whole blood ionized calcium levels significantly at this time point (Fig. 1). There was a tendency for ionized calcium levels to return toward or to normal for all treatments except 1,25(OH)2D3 by day 5. The day 5 ionized calcium levels were determined at sacrifice, 12 h postinjection of cytokine or hormone and decreases in levels from day 3 are possibly due to the different time of calcium measurement from injection. The elevation in ionized calcium levels was smallest with IL-1β and greatest with 1,25(OH)2D3. Treatment with cOPG (2.5 mg/kg/day) in combination with all the above factors maintained whole blood ionized calcium levels in the normal range at all time points. Injections of cOPG had no effect on calcium levels in vehicle-treated control mice. Additionally, mice on a low calcium diet, treated with vehicle, maintained normal calcium levels probably by mobilizing bone calcium. However, when they were treated concurrently with cOPG (2.5 mg/kg/day) they became hypocalcemic (Fig. 5).
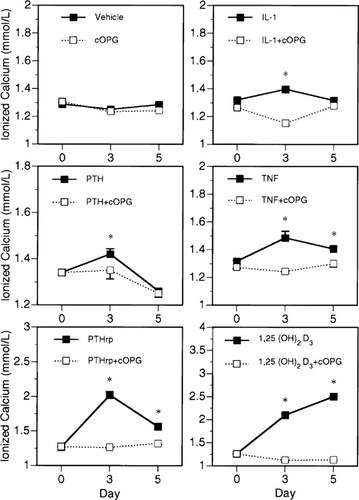
Effects of cOPG on whole blood ionized calcium levels in mice treated with various bone resorption stimulators. Treatment with IL-1β (5 μg/day), TNF-α (50 μg/day), PTH (100 μg/day), PTHrP (20 μg/day), or 1,25(OH)2D3 (0.5 μg/day) induced a significant elevation of whole blood ionized calcium levels in mice by day 3 and tended toward normal by day 5 in all groups except 1,25(OH)2D3. Concurrent treatment with cOPG (2.5 mg/kg/day) maintained ionized calcium levels in the normal range at all time points. Ionized calcium levels were determined prior to initiating any treatment on day 0 and 3 h postcytokine or hormone injection on day 3. Day 5 levels were determined 12 h postinjection of cytokine or hormone. *Different from cOPG treatment group, p < 0.05 (group n = 5, mean ± SE).
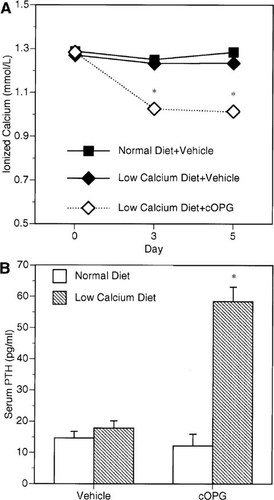
The effects of cOPG on mice fed a low calcium diet. Whole blood ionized calcium levels (A) and PTH serum levels (B) were measured in mice fed either low (0.02% calcium) or high (0.60% calcium). Treatment with cOPG blocks osteoclastic bone resorption and induces significant hypocalcemia in mice on a low calcium diet (p < 0.001). PTH serum levels are significantly increased in animals on a low calcium diet treated concurrently with cOPG for 5 days (p < 0.001) indicating an intact parathyroid gland response to decreased calcium levels. Animals on normal diet showed no significant change in whole blood ionized calcium or serum PTH levels with or without cOPG treatment. *Different from vehicle-treated control, p < 0.001 (group n = 5, mean ± SE).
Bone resorption and osteoclast numbers are decreased with cOPG treatment
Each cytokine, hormone treatment, or low calcium diet induced a significant elevation of bone resorption evident radiographically as zones of increased radiolucency in the calvaria. Histologically, these changes were associated with an increase in the size of marrow spaces and numbers of osteoclasts. Figure 2 shows the effects of IL-1β on the radiographic and histologic appearance of these changes, which were similar to those produced by the other treatments (data not shown). The exception to this pattern was PTHrP, which also induced marked periosteal bone resorption on the dorsal calvarial surface. cOPG inhibited these changes for all treatments (Fig. 2). Osteoclast numbers were increased significantly above vehicle-treated animals by each of the treatments (Fig. 3). In each case, however, cOPG treatment (2.5 mg/kg/day) decreased these values to within or below the normal range. cOPG treatment also reduced osteoclast numbers in control animals by 51%.
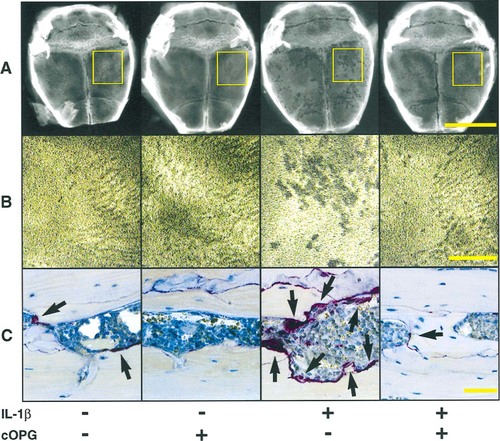
Effects of cOPG on the radiographic and histologic changes seen in calvaria of mice treated with (+) or without (−) IL-1β. (A) Calvarial radiographs enlarged to show regional effects of cytokine treatment. The majority of bone pitting is localized to the area of injection (yellow rectangle); however, an effect of treatment over the entire bone surface is noted. (B) Magnification of the cytokine injection region (yellow rectangle) shows increased bone changes in IL-1β–treated animals (+/−) compared with vehicle-treated mice (−/−) which is absent in cOPG treated mice (+/+). (C) Photomicrographs of the TRAP-stained sections through the calvaria at the site of cytokine injection shows increased osteoclastic bone resorption, which corresponds to the bone changes seen radiographically. cOPG blocks the increased bone resorption caused by IL-1β injections. Arrowheads indicate osteoclasts.
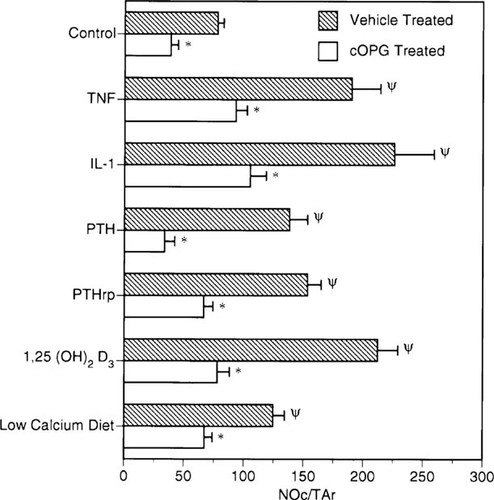
Effects of cOPG on osteoclast stimulation by various bone resorption stimulators. Osteoclast numbers per unit bone tissue area (Oc.N/TAr) were elevated in the calvaria of mice treated with bone resorption stimulators (see figure for list) and in mice on a low calcium diet (0.02% vs. 0.60% calcium) after 4 days. cOPG (2.5 mg/kg/day) treatments reduced osteoclast numbers in all challenges relative to vehicle treatments to a level similar to vehicle-treated control mice. cOPG also reduced osteoclast numbers in vehicle-treated mice receiving a normal diet. Different from vehicle-treated control p < 0.001. *Different from treatment group without cOPG, p < 0.001 (group n = 5, mean ± SE).
Weight loss due to hypercalcemia is inhibited
Marked weight loss was seen in mice treated with either IL-1β, TNF-α, or 1,25(OH)2D3 and was as great as 29% with IL-1β by day 5 (Fig. 4). Weight loss associated with IL-1β and TNF-α treatment was not inhibited by cOPG therapy, indicating that weight loss was likely due to inflammatory effects and muscle wasting associated with these cytokines and not the modest hypercalcemia.22, 23 Animals treated with 1,25(OH)2D3 alone also showed severe weight loss that was accompanied by profound hypercalcemia. This weight loss was completely inhibited with daily cOPG therapy (Fig. 4), which normalized calcium levels, indicating that the weight loss was probably a result of the hypercalcemia in these animals. No significant weight loss was observed for PTH, PTHrP, and low calcium diet-treated groups (data not shown).
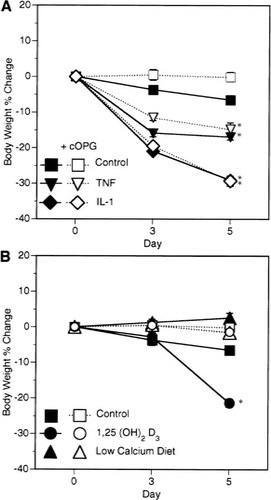
Effect of cOPG (2.5 mg/kg/day) treatment on weight loss due to IL-1β (5 μg/day), TNF-α (50 μg/day), and 1,25(OH)2D3 (0.5 μg/day) treatments. (A) Weight loss induced by TNF-α and IL-1β is not prevented by cOPG. (B) Weight loss induced by 1,25(OH)2D3 treatment is completely prevented by cOPG treatment. *Different from vehicle-treated control, p < 0.001 (group n = 5, mean ± SE).
Serum PTH levels are increased in animals on a low calcium diet treated with cOPG
To determine the effect of cOPG therapy on calcium homeostasis, we treated animals on a low calcium diet for 5 days, and analyzed whole blood ionized calcium levels and serum PTH levels (Fig. 5). Whole blood ionized calcium levels were maintained in the normal range for animals on a normal diet (0.60% calcium) treated with and without cOPG, 1.18 ± 0.01 mmol/l and 1.20 ± 0.01 mmol/l, respectively (Fig. 5). Animals on a low calcium (0.02%) diet treated with vehicle also had calcium levels in the normal range, 1.18 ± 0.01 mmol/l (Fig. 5). However, treatment of animals on a low calcium diet with cOPG (2.5 mg/kg/day) for 5 days induced significant hypocalcemia, decreasing whole blood ionized calcium levels to 1.09 ± 0.01 mmol/l (p < 0.001) (Fig. 5). Serum PTH levels were similar in the mice on normal diet with and without OPG treatment (Fig. 5). However, in mice on low calcium diet treated with cOPG, PTH serum levels markedly increased over vehicle-treated mice from 14.6 ± 2.18 pg/ml to 58.3 ± 4.68 pg/ml, respectively (p < 0.001). Thus, it appears that cOPG blocks the bone-calcium mobilizing effects of increased circulating PTH in animals fed a low calcium diet.
DISCUSSION
cOPG inhibited the hypercalcemia, radiographic evidence of increased bone resorption, and increased osteoclast numbers produced by each of the hormones and cytokines tested. In addition, cOPG produced hypocalcemia and inhibited increased bone resorption and osteoclast numbers in mice maintained on a low calcium diet. Thus, cOPG appears to be an antiresorptive protein with broad activity against the hormones and cytokines known to induce bone resorption.
The probable mechanism of action for cOPG is that it binds to OPG ligand4 (also known as TRANCE,5 RANKL,6 and ODF7) and prevents its interaction with a membrane bound receptor (possibly RANK6) on osteoclasts and preosteoclasts. OPG ligand has been proposed to be the long-sought factor produced by cells of the osteoblast lineage in response to the action of hormones and cytokines to mediate their bone resorbing effects.4, 7 Expression of OPG ligand is regulated by bone-resorptive agents such as prostaglandin E2, PTH, IL-11, and 1,25(OH)2D3)7 and is able to replace the requirement for osteoblasts or stromal cells in in vitro models of osteoclast differentiation.4 The ability of cOPG to inhibit the resorptive effects of all the factors and conditions tested supports a role for OPG ligand in mediating both physiologic and pathologic signals that serve to increase bone resorption. While recombinant OPG ligand can increase blood calcium levels in normal mice dose dependently,4 it is unclear whether the factors tested regulate OPG ligand levels locally to increase bone resorption or whether OPG ligand is an essential permissive factor. Pilot studies (data not shown) showed that PTH, PTHrP, 1,25(OH)2D3, TNF-α, and IL-1β each produced significant hypercalcemia, which varied from mild to severe, at the doses chosen for the current study. The doses and the hypercalcemia induced by PTH, PTHrP, and IL-1β are similar to those previously reported for PTH and PTHrP12 and IL-1α.20 The concurrent increases in osteoclast numbers indicate that, at least in part, the hypercalcemia was mediated by elevated bone resorption. PTH and PTHrP have the additional mechanism of increasing renal calcium reabsorption, and this may have contributed to their hypercalcemic effects.24, 25 In addition to effects on bone resorption,26, 27 1,25(OH)2D3 is known to increase intestinal calcium absorption13; however, the contribution of calcium from this source would have been minimal as the mice receiving 1,25(OH)2D3 were maintained on a low calcium diet prior to initiating treatment. The hypocalcemia and decrease in osteoclast numbers observed with cOPG treatment of mice on a low calcium diet, despite high endogenous PTH levels, is support that cOPG blocks the action of endogenous PTH on bone resorption. It is likely that mobilization of calcium from bone is required to maintain normal blood calcium levels in these calcium-deprived mice. However, the effects of cOPG on renal calcium reabsorption have not been investigated, and an additional renal effect cannot be ruled out.
Bone resorption was increased in the calvaria of mice injected with PTH, PTHrP, TNF-α, IL-1β, and 1,25(OH)2D3, as indicated by increased radiolucency of the calvaria (Fig. 2) and increased osteoclast number (Fig. 3). For all the treatments, except PTHrP, resorption was increased predominantly on the endosteal surfaces of the calvaria. PTHrP was the only treatment that also increased bone resorption on the dorsal periosteal calvarial surface. This difference may reflect different tissue residence time or different ability to diffuse through the periosteum into the endosteal spaces. However, for each treatment, cOPG was able to inhibit the increases in both bone resorption and osteoclast number.
Treatment with 1,25(OH)2D3, TNF-α, and IL-1β each produced weight loss in the mice, but cOPG was able to inhibit this effect for only 1,25(OH)2D3. In humans, hypercalcemia has been correlated with weight loss, possibly due to dehydration.28 The weight loss induced by 1,25(OH)2D3 in this study was most likely secondary to the profound hypercalcemia because the weight loss was inhibited by concurrent treatment with cOPG which normalized the calcium levels. A less likely possibility is that osteoclasts activated by 1,25(OH)2D3 treatment expressed inflammatory cytokines that contributed to the weight loss29 and cOPG inhibited this indirect effect by blocking increases in osteoclast numbers. The weight loss induced by TNF-α and IL-1β appeared unrelated to calcium levels because the hypercalcemia induced was mild and not influenced by maintenance of normal calcium levels by cOPG. The weight loss with these cytokines probably reflects a inflammatory response and skeletal muscle catabolism.22, 23 cOPG does not bind or directly inhibit TNF-α (W.J. Boyle, unpublished data), even though TNF-α and OPG ligand share structural homology.5
All of the factors tested have been implicated in human bone disease associated with up-regulated osteoclastic bone resorption. TNF-α and IL-1β have been found at increased levels associated with postmenopausal osteoporosis30-32 and are associated with inflammatory diseases such as rheumatoid arthritis that produce localized bone loss.33, 34 Increases in PTH levels are associated with osteoitis fibrosa in renal failure patients24, 25, 35 and hypercalcemia in patients with primary hyperparathyroidism.36 1,25(OH)2D3 overproduction can produce hypercalcemia in patients with sarcoid.37, 38 PTHrP has been shown to be secreted by both metastatic and nonmetastatic solid tumors as well as by hematological malignancies and is considered to be the most common cause of humoral hypercalcemia of malignancy.39, 40 In this study, only the short-term antiresorptive effects of cOPG are examined, and sustained effects were not evaluated. However, in transgenic animals over-expressing OPG, osteopetrosis is sustained and bone resorption is inhibited for at least 10 weeks, indicating that sustained activity of OPG is possible.1
In conclusion, the broad spectrum of activity of cOPG in inhibiting the resorptive activity of the hormones and cytokines tested is indicative of the central importance of OPG/OPGL signaling in the regulation of bone resorption. cOPG, a protein with specific effects on osteoclast differentiation and activation, is a potential therapeutic for the treatment of bone diseases associated with increased bone resorption.
Acknowledgements
The authors thank Diane Duryea and Carol Burgh of the Amgen, Inc. Pathology Department for expert technical assistance, and William J. Boyle for critical review in preparation of this manuscript.




